Ras/MAPK signaling from endomembranes
- PMID: 19615955
- PMCID: PMC3003591
- DOI: 10.1016/j.molonc.2009.06.004
Ras/MAPK signaling from endomembranes
Abstract
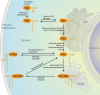
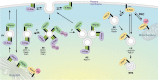
Signal transduction along the Ras/MAPK pathway has been generally thought to take place at the plasma membrane. It is now evident that the plasma membrane is not the only platform capable of Ras/MAPK signal induction. Fusion of Ras with green fluorescent protein and the development of genetically encoded fluorescent probes for Ras activation have revealed signaling events on a variety of intracellular membranes including endosomes, the Golgi apparatus and the endoplasmic reticulum. Thus, the Ras/MAPK pathway is spatially compartmentalized within cells and this may afford greater complexity of signal output.
Figures
Similar articles
-
Ras pathway signaling on endomembranes.Curr Opin Cell Biol. 2003 Apr;15(2):136-42. doi: 10.1016/s0955-0674(03)00016-4. Curr Opin Cell Biol. 2003. PMID: 12648668 Review.
-
Compartmentalized Ras/MAPK signaling.Annu Rev Immunol. 2006;24:771-800. doi: 10.1146/annurev.immunol.24.021605.090723. Annu Rev Immunol. 2006. PMID: 16551266 Review.
-
Intracellular signaling: peripatetic Ras.Curr Biol. 2009 Jun 9;19(11):R454-7. doi: 10.1016/j.cub.2009.04.045. Curr Biol. 2009. PMID: 19515353
-
Ras signaling on the Golgi.Curr Opin Cell Biol. 2006 Apr;18(2):162-7. doi: 10.1016/j.ceb.2006.02.004. Epub 2006 Feb 20. Curr Opin Cell Biol. 2006. PMID: 16488589 Review.
-
Compartmentalized Ras signaling differentially contributes to phenotypic outputs.Cell Signal. 2013 Sep;25(9):1748-53. doi: 10.1016/j.cellsig.2013.05.004. Epub 2013 May 22. Cell Signal. 2013. PMID: 23707528 Free PMC article.
Cited by
-
Ubiquitination: Added complexity in Ras and Rho family GTPase function.Small GTPases. 2011 Jul;2(4):192-201. doi: 10.4161/sgtp.2.4.16707. Epub 2011 Jul 1. Small GTPases. 2011. PMID: 22145091 Free PMC article.
-
Targeting PI3K/AKT/mTOR and MAPK Signaling Pathways in Gastric Cancer.Int J Mol Sci. 2024 Feb 3;25(3):1848. doi: 10.3390/ijms25031848. Int J Mol Sci. 2024. PMID: 38339127 Free PMC article. Review.
-
Rab GTPases implicated in inherited and acquired disorders.Semin Cell Dev Biol. 2011 Feb;22(1):57-68. doi: 10.1016/j.semcdb.2010.12.005. Epub 2010 Dec 13. Semin Cell Dev Biol. 2011. PMID: 21147240 Free PMC article. Review.
-
Subcellular Distribution of S-Nitrosylated H-Ras in Differentiated and Undifferentiated PC12 Cells during Hypoxia.Cell J. 2017 Oct;19(3):443-451. doi: 10.22074/cellj.2017.4546. Epub 2017 Aug 19. Cell J. 2017. PMID: 28836406 Free PMC article.
-
Nonredundant functions for Ras GTPase-activating proteins in tissue homeostasis.Sci Signal. 2013 Feb 26;6(264):re1. doi: 10.1126/scisignal.2003669. Sci Signal. 2013. PMID: 23443682 Free PMC article. Review.
References
-
- Barbacid, M. , 1987. ras Genes. Ann. Rev. Biochem.. 56, 779–827. - PubMed
-
- Bivona, T.G. , Perez De Castro, I. , Ahearn, I.M. , Grana, T.M. , Chiu, V.K. , Lockyer, P.J. , Cullen, P.J. , Pellicer, A. , Cox, A.D. , Philips, M.R. , 2003. Phospholipase Cγ activates Ras on the Golgi apparatus by means of RasGRP1. Nature. 424, 694–698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

