A novel DNA vaccine containing multiple TB-specific epitopes casted in a natural structure (ECANS) confers protective immunity against pulmonary mycobacterial challenge
- PMID: 19615961
- PMCID: PMC7115364
- DOI: 10.1016/j.vaccine.2009.06.093
A novel DNA vaccine containing multiple TB-specific epitopes casted in a natural structure (ECANS) confers protective immunity against pulmonary mycobacterial challenge
Abstract
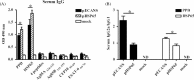
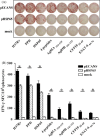
Epitope-based DNA vaccines designed to induce T cell responses specific for Mycobacterium tuberculosis (M. tb) are being developed as a means of addressing vaccine potency. In this study, we predicted 4 T cell epitopes from ESAT-6, Ag85A/B and CFP-10 antigens and constructed an ECANS (epitopes casted in a natural structure) DNA vaccine by inserting the epitope DNA segments separately into the gene backbone of M. tb-derived HSP65 (heat shock protein 65) carrier. The immunogenicity and protective efficacy of pECANS DNA vaccine were assessed in BALB/c mice after intramuscular immunization with 4 doses of 50 microg ECANS DNA and followed by mycobaterial challenge 4 weeks after the last immunization. Compared to plasmid encoding HSP65, pECANS DNA immunization elicited remarkably higher levels of IFN-gamma production by both CD4(+) and CD8(+) T cells, which were coupled with higher frequencies of antigen-specific T cells and higher CTL activity. Significantly enhanced levels of Th1 cytokines (IFN-gamma and IL-12) and increased serum IgG2a/IgG1 ratio were also noted, indicating a predominant Th1 immune response achieved by pECANS DNA immunization. In the consequence, a better protection against Mycobacterium bovis BCG challenge was achieved which was evidenced by reduced bacterial loads in lungs and spleens and profound attenuation of lung inflammation and injury. Our results suggested that multi-T cell-epitope based ECANS gene vaccine induced T cell response to multiple T cell epitopes and led to enhanced protection against mycobacterial challenge. This strategy might be a useful platform to design multi-T cell epitope-based vaccine against M. tb infection.
Figures





References
-
- Maartens G., Wilkinson R.J. Tuberculosis. Lancet. 2007;370:2030–2043. - PubMed
-
- Thom M., Howard C., Villarreal-Ramos B., Mead E., Vordermeier M., Hope J. Consequence of prior exposure to environmental mycobacteria on BCG vaccination and diagnosis of tuberculosis infection. Tuberculosis (Edinb) 2008;88:324–334. - PubMed
-
- Brave A., Boberg A., Gudmundsdotter L., Rollman E., Hallermalm K., Ljungberg K. A new multi-clade DNA prime/recombinant MVA boost vaccine induces broad and high levels of HIV-1-specific CD8(+) T-cell and humoral responses in mice. Mol Ther. 2007;15:1724–1733. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

