Dynamics of galectin-3 in the nucleus and cytoplasm
- PMID: 19616076
- PMCID: PMC2815258
- DOI: 10.1016/j.bbagen.2009.07.005
Dynamics of galectin-3 in the nucleus and cytoplasm
Abstract
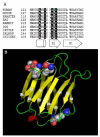
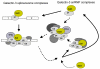
This review summarizes selected studies on galectin-3 (Gal3) as an example of the dynamic behavior of a carbohydrate-binding protein in the cytoplasm and nucleus of cells. Within the 15-member galectin family of proteins, Gal3 (M(r) approximately 30,000) is the sole representative of the chimera subclass in which a proline- and glycine-rich NH(2)-terminal domain is fused onto a COOH-terminal carbohydrate recognition domain responsible for binding galactose-containing glycoconjugates. The protein shuttles between the cytoplasm and nucleus on the basis of targeting signals that are recognized by importin(s) for nuclear localization and exportin-1 (CRM1) for nuclear export. Depending on the cell type, specific experimental conditions in vitro, or tissue location, Gal3 has been reported to be exclusively cytoplasmic, predominantly nuclear, or distributed between the two compartments. The nuclear versus cytoplasmic distribution of the protein must reflect, then, some balance between nuclear import and export, as well as mechanisms of cytoplasmic anchorage or binding to a nuclear component. Indeed, a number of ligands have been reported for Gal3 in the cytoplasm and in the nucleus. Most of the ligands appear to bind Gal3, however, through protein-protein interactions rather than through protein-carbohydrate recognition. In the cytoplasm, for example, Gal3 interacts with the apoptosis repressor Bcl-2 and this interaction may be involved in Gal3's anti-apoptotic activity. In the nucleus, Gal3 is a required pre-mRNA splicing factor; the protein is incorporated into spliceosomes via its association with the U1 small nuclear ribonucleoprotein (snRNP) complex. Although the majority of these interactions occur via the carbohydrate recognition domain of Gal3 and saccharide ligands such as lactose can perturb some of these interactions, the significance of the protein's carbohydrate-binding activity, per se, remains a challenge for future investigations.
Copyright 2009 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Nucleocytoplasmic Shuttling of Porcine Parvovirus NS1 Protein Mediated by the CRM1 Nuclear Export Pathway and the Importin α/β Nuclear Import Pathway.J Virol. 2022 Jan 12;96(1):e0148121. doi: 10.1128/JVI.01481-21. Epub 2021 Oct 13. J Virol. 2022. PMID: 34643426 Free PMC article.
-
Transport of galectin-3 between the nucleus and cytoplasm. II. Identification of the signal for nuclear export.Glycobiology. 2006 Jul;16(7):612-22. doi: 10.1093/glycob/cwj089. Epub 2006 Feb 9. Glycobiology. 2006. PMID: 16473834
-
Type 1 parathyroid hormone receptor (PTH1R) nuclear trafficking: regulation of PTH1R nuclear-cytoplasmic shuttling by importin-alpha/beta and chromosomal region maintenance 1/exportin 1.Endocrinology. 2007 May;148(5):2282-9. doi: 10.1210/en.2007-0157. Epub 2007 Feb 22. Endocrinology. 2007. PMID: 17317770
-
Nucleocytoplasmic lectins.Biochim Biophys Acta. 2004 Jul 6;1673(1-2):75-93. doi: 10.1016/j.bbagen.2004.03.013. Biochim Biophys Acta. 2004. PMID: 15238251 Review.
-
Nuclear transport of galectin-3 and its therapeutic implications.Semin Cancer Biol. 2014 Aug;27:30-8. doi: 10.1016/j.semcancer.2014.03.004. Epub 2014 Mar 19. Semin Cancer Biol. 2014. PMID: 24657939 Free PMC article. Review.
Cited by
-
Advances in Visualizing Microglial Cells in Human Central Nervous System Tissue.Biomolecules. 2022 Apr 19;12(5):603. doi: 10.3390/biom12050603. Biomolecules. 2022. PMID: 35625531 Free PMC article. Review.
-
What Happens If a Human Galectin Enters the Endoplasmic Reticulum?Methods Mol Biol. 2022;2442:247-288. doi: 10.1007/978-1-0716-2055-7_15. Methods Mol Biol. 2022. PMID: 35320531
-
Extracellular and intracellular small-molecule galectin-3 inhibitors.Sci Rep. 2019 Feb 18;9(1):2186. doi: 10.1038/s41598-019-38497-8. Sci Rep. 2019. PMID: 30778105 Free PMC article.
-
Polyamidoamine Dendrimer Conjugates with Cyclodextrins as Novel Carriers for DNA, shRNA and siRNA.Pharmaceutics. 2012 Feb 1;4(1):130-48. doi: 10.3390/pharmaceutics4010130. Pharmaceutics. 2012. PMID: 24300184 Free PMC article.
-
Effect of galectin-3 on the behavior of Eca‑109 human esophageal cancer cells.Mol Med Rep. 2015 Feb;11(2):896-902. doi: 10.3892/mmr.2014.2873. Epub 2014 Nov 5. Mol Med Rep. 2015. PMID: 25373317 Free PMC article.
References
-
- Barondes SH, Castronovo V, Cooper DW, Cummings RD, Drickamer K, Feizi T, et al. Galectins: a family of animal β-galactoside-binding lectins. Cell. 1994;76:597–598. - PubMed
-
- Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj. J. 2004;19:433–440. - PubMed
-
- Rini JM, Lobsanov YD. New animal lectin structures. Curr. Opin. Struct. Biol. 1999;9:578–584. - PubMed
-
- Bachhawat-Sikder K, Thomas CJ, Surolia A. Thermodynamic analysis of the binding of galactose and poly-N-acetyllactosamine derivatives to human galectin-3. FEBS Lett. 2001;500:75–79. - PubMed
-
- Hsu DK, Zuberi RI, Liu F-T. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J. Biol. Chem. 1992;267:14167–14174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

