Dynamics of galectin-3 in the nucleus and cytoplasm
- PMID: 19616076
- PMCID: PMC2815258
- DOI: 10.1016/j.bbagen.2009.07.005
Dynamics of galectin-3 in the nucleus and cytoplasm
Abstract
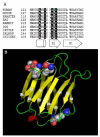
This review summarizes selected studies on galectin-3 (Gal3) as an example of the dynamic behavior of a carbohydrate-binding protein in the cytoplasm and nucleus of cells. Within the 15-member galectin family of proteins, Gal3 (M(r) approximately 30,000) is the sole representative of the chimera subclass in which a proline- and glycine-rich NH(2)-terminal domain is fused onto a COOH-terminal carbohydrate recognition domain responsible for binding galactose-containing glycoconjugates. The protein shuttles between the cytoplasm and nucleus on the basis of targeting signals that are recognized by importin(s) for nuclear localization and exportin-1 (CRM1) for nuclear export. Depending on the cell type, specific experimental conditions in vitro, or tissue location, Gal3 has been reported to be exclusively cytoplasmic, predominantly nuclear, or distributed between the two compartments. The nuclear versus cytoplasmic distribution of the protein must reflect, then, some balance between nuclear import and export, as well as mechanisms of cytoplasmic anchorage or binding to a nuclear component. Indeed, a number of ligands have been reported for Gal3 in the cytoplasm and in the nucleus. Most of the ligands appear to bind Gal3, however, through protein-protein interactions rather than through protein-carbohydrate recognition. In the cytoplasm, for example, Gal3 interacts with the apoptosis repressor Bcl-2 and this interaction may be involved in Gal3's anti-apoptotic activity. In the nucleus, Gal3 is a required pre-mRNA splicing factor; the protein is incorporated into spliceosomes via its association with the U1 small nuclear ribonucleoprotein (snRNP) complex. Although the majority of these interactions occur via the carbohydrate recognition domain of Gal3 and saccharide ligands such as lactose can perturb some of these interactions, the significance of the protein's carbohydrate-binding activity, per se, remains a challenge for future investigations.
Copyright 2009 Elsevier B.V. All rights reserved.
Figures
References
-
- Barondes SH, Castronovo V, Cooper DW, Cummings RD, Drickamer K, Feizi T, et al. Galectins: a family of animal β-galactoside-binding lectins. Cell. 1994;76:597–598. - PubMed
-
- Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj. J. 2004;19:433–440. - PubMed
-
- Rini JM, Lobsanov YD. New animal lectin structures. Curr. Opin. Struct. Biol. 1999;9:578–584. - PubMed
-
- Bachhawat-Sikder K, Thomas CJ, Surolia A. Thermodynamic analysis of the binding of galactose and poly-N-acetyllactosamine derivatives to human galectin-3. FEBS Lett. 2001;500:75–79. - PubMed
-
- Hsu DK, Zuberi RI, Liu F-T. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J. Biol. Chem. 1992;267:14167–14174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

