PET radiotracers: crossing the blood-brain barrier and surviving metabolism
- PMID: 19616318
- PMCID: PMC2805092
- DOI: 10.1016/j.tips.2009.05.005
PET radiotracers: crossing the blood-brain barrier and surviving metabolism
Abstract
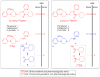
Radiotracers for imaging protein targets in the living human brain with positron emission tomography (PET) are increasingly useful in clinical research and in drug development. Such radiotracers must fulfill many criteria, among which an ability to enter brain adequately and reversibly without contamination by troublesome radiometabolites is desirable for accurate measurement of the density of a target protein (e.g. neuroreceptor, transporter, enzyme or plaque). Candidate radiotracers can fail as a result of poor passive brain entry, rejection from brain by efflux transporters or undesirable metabolism. These issues are reviewed. Emerging PET radiotracers for measuring efflux transporter function and new strategies for ameliorating radiotracer metabolism are discussed. A growing understanding of the molecular features affecting the brain penetration, metabolism and efflux transporter sensitivity of prospective radiotracers should ultimately lead to their more rational and efficient design, and also to their greater efficacy.
Figures

References
-
- Jacobs AH, et al. PET-based molecular imaging in neuroscience. Eur J Nucl Med & Mol Imaging. 2003;30:1051–1065. - PubMed
-
- Lee CM, Farde L. Using positron emission tomography to facilitate drug development. TiPs. 2006;27:310–316. - PubMed
-
- Fowler JS, Ido T. In: Handbook of Radiopharmaceuticals, Radiochemistry and Applications. Welch MJ, Redvanly CS, editors. Chapter 9. Wiley; Chichester: 2003. pp. 307–321.
-
- Pike VW. Positron-emitting radioligands for studies in vivo Probes for human psychopharmacology. J Psychopharmacology. 1993;7:139–158. - PubMed
-
- Laruelle M, et al. Relationships between radiotracer properties and image quality in molecular imaging of the brain with positron emission tomography. Mol Imaging Biol. 2003;5:363–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

