Investigations into pulsed high-intensity focused ultrasound-enhanced delivery: preliminary evidence for a novel mechanism
- PMID: 19616368
- PMCID: PMC2752481
- DOI: 10.1016/j.ultrasmedbio.2009.04.020
Investigations into pulsed high-intensity focused ultrasound-enhanced delivery: preliminary evidence for a novel mechanism
Abstract
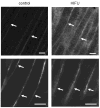
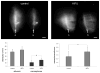
Pulsed high-intensity focused ultrasound (HIFU) exposures without ultrasound contrast agents have been used for noninvasively enhancing the delivery of various agents to improve their therapeutic efficacy in a variety of tissue models in a nondestructive manner. Despite the versatility of these exposures, little is known about the mechanisms by which their effects are produced. In this study, pulsed-HIFU exposures were given in the calf muscle of mice, followed by the administration of a variety of fluorophores, both soluble and particulate, by local or systemic injection. In vivo imaging (whole animal and microscopic) was used to quantify observations of increased extravasation and interstitial transport of the fluorophores as a result of the exposures. Histological analysis indicated that the exposures caused some structural alterations such as enlarged gaps between muscle fiber bundles. These effects were consistent with increasing the permeability of the tissues; however, they were found to be transient and reversed themselves gradually within 72 h. Simulations of radiation force-induced displacements and the resulting local shear strain they produced were carried out to potentially explain the manner by which these effects occurred. A better understanding of the mechanisms involved with pulsed HIFU exposures for noninvasively enhancing delivery will facilitate the process for optimizing their use.
Figures






References
-
- Baker BA, Mercer RR, Geronilla KB, Kashon ML, Miller GR, Cutlip RG. Stereological analysis of muscle morphology following exposure to repetitive stretch-shortening cycles in a rat model. Appl Physiol Nutr Metab. 2006;31:167–79. - PubMed
-
- Baker BA, Mercer RR, Geronilla KB, Kashon ML, Miller GR, Cutlip RG. Impact of repetition number on muscle performance and histological response. Med Sci Sports Exerc. 2007;39:1275–81. - PubMed
-
- Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396–409. - PubMed
-
- Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–69. - PubMed
-
- Clement GT. Perspectives in clinical uses of high-intensity focused ultrasound. Ultrasonics. 2004;42:1087–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

