Interleukin-32 positively regulates radiation-induced vascular inflammation
- PMID: 19616744
- PMCID: PMC2713876
- DOI: 10.1016/j.ijrobp.2009.04.017
Interleukin-32 positively regulates radiation-induced vascular inflammation
Abstract
Purpose: To study the role of interleukin-32 (IL-32), a novel protein only detected in human tissues, in ionizing radiation (IR)-induced vascular inflammation.
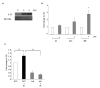
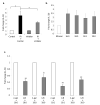
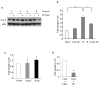
Methods and materials: Irradiated (0-6 Gy) human umbilical vein endothelial cells treated with or without various agents--a cytosolic phospholipase A2 (cPLA2) inhibitor, a cyclooxygenase-2 (Cox-2) inhibitor, or lysophosphatidylcholines (LPCs)--were used to assess IL-32 expression by Northern blot analysis and quantitative reverse transcriptase-polymerase chain reaction. Expression of cell adhesion molecules and leukocyte adhesion to endothelial cells using human acute monocytic leukemia cell line (THP-1) cells was also analyzed.
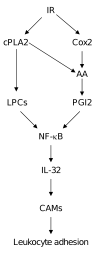
Results: Ionizing radiation dramatically increased IL-32 expression in vascular endothelial cells through multiple pathways. Ionizing radiation induced IL-32 expression through nuclear factor kappaB activation, through induction of cPLA2 and LPC, as well as induction of Cox-2 and subsequent conversion of arachidonic acid to prostacyclin. Conversely, blocking nuclear factor kappaB, cPLA2, and Cox-2 activity impaired IR-induced IL-32 expression. Importantly, IL-32 significantly enhanced IR-induced expression of vascular cell adhesion molecules and leukocyte adhesion on endothelial cells.
Conclusion: This study identifies IL-32 as a positive regulator in IR-induced vascular inflammation, and neutralization of IL-32 may be beneficial in protecting from IR-induced inflammation.
Conflict of interest statement
Figures

References
-
- Movsas B, Raffin TA, Epstein AH, et al. Pulmonary radiation injury. Chest. 1997;111:1061–1076. - PubMed
-
- Hallahan DE, Virudachalam S. Ionizing radiation mediates expression of cell adhesion molecules in distinct histological patterns within the lung. Cancer Res. 1997;57:2096–2099. - PubMed
-
- Hallahan DE, Virudachalam S, Kuchibhotla J. Nuclear factor kappaB dominant negative genetic constructs inhibit X-ray induction of cell adhesion molecules in the vascular endothelium. Cancer Res. 1998;58:5484–5488. - PubMed
-
- Kim SH, Han SY, Azam T, et al. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22:131–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

