A cellular restriction dictates the permissivity of nondividing monocytes/macrophages to lentivirus and gammaretrovirus infection
- PMID: 19616766
- PMCID: PMC2777639
- DOI: 10.1016/j.chom.2009.05.022
A cellular restriction dictates the permissivity of nondividing monocytes/macrophages to lentivirus and gammaretrovirus infection
Abstract
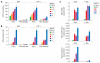
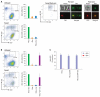
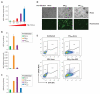
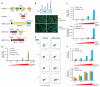
Primate lentiviruses, including HIV-1, transduce terminally differentiated, nondividing myeloid cells; however, these cells are refractory to infection by gammaretroviruses such as murine leukemia virus (MLV). Here, we present evidence that a cellular restriction is the obstacle to transduction of macrophages by MLV. Neutralization of the restriction by Vpx, a primate lentiviral protein previously shown to protect primate lentiviruses from a macrophage restriction, rendered macrophages permissive to MLV infection. We further demonstrate that this restriction prevents transduction of quiescent monocytes by HIV-1. Monocyte-HeLa heterokaryons were resistant to HIV-1 infection, while heterokaryons formed between monocytes and HeLa cells expressing Vpx were permissive to HIV-1 infection. Encapsidation of Vpx within HIV-1 virions conferred the ability to infect quiescent monocytes. Collectively, our results indicate that the relative ability of lentiviruses and gammaretroviruses to transduce nondividing myeloid cells is dependent upon their ability to neutralize a cellular restriction.
Figures


Similar articles
-
Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction.PLoS Pathog. 2008 May 2;4(5):e1000057. doi: 10.1371/journal.ppat.1000057. PLoS Pathog. 2008. PMID: 18451984 Free PMC article.
-
Human immunodeficiency virus type 1 modified to package Simian immunodeficiency virus Vpx efficiently infects macrophages and dendritic cells.J Virol. 2011 Jul;85(13):6263-74. doi: 10.1128/JVI.00346-11. Epub 2011 Apr 20. J Virol. 2011. PMID: 21507971 Free PMC article.
-
Host SAMHD1 protein restricts endogenous reverse transcription of HIV-1 in nondividing macrophages.Retrovirology. 2018 Oct 13;15(1):69. doi: 10.1186/s12977-018-0452-z. Retrovirology. 2018. PMID: 30316304 Free PMC article.
-
Host hindrance to HIV-1 replication in monocytes and macrophages.Retrovirology. 2010 Apr 7;7:31. doi: 10.1186/1742-4690-7-31. Retrovirology. 2010. PMID: 20374633 Free PMC article. Review.
-
Limelight on two HIV/SIV accessory proteins in macrophage infection: is Vpx overshadowing Vpr?Retrovirology. 2010 Apr 9;7:35. doi: 10.1186/1742-4690-7-35. Retrovirology. 2010. PMID: 20380700 Free PMC article. Review.
Cited by
-
SAMHD1 restricts HIV-1 cell-to-cell transmission and limits immune detection in monocyte-derived dendritic cells.J Virol. 2013 Mar;87(5):2846-56. doi: 10.1128/JVI.02514-12. Epub 2012 Dec 26. J Virol. 2013. PMID: 23269793 Free PMC article.
-
Aicardi-Goutieres syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase.J Biol Chem. 2011 Dec 23;286(51):43596-43600. doi: 10.1074/jbc.C111.317628. Epub 2011 Nov 7. J Biol Chem. 2011. PMID: 22069334 Free PMC article.
-
β-Catenin/TCF-4 signaling regulates susceptibility of macrophages and resistance of monocytes to HIV-1 productive infection.Curr HIV Res. 2014;12(3):164-73. doi: 10.2174/1570162x12666140526122249. Curr HIV Res. 2014. PMID: 24862328 Free PMC article.
-
Evolutionary and functional analyses of the interaction between the myeloid restriction factor SAMHD1 and the lentiviral Vpx protein.Cell Host Microbe. 2012 Feb 16;11(2):205-17. doi: 10.1016/j.chom.2012.01.007. Epub 2012 Feb 1. Cell Host Microbe. 2012. PMID: 22305291 Free PMC article.
-
SAMHD1-dependent retroviral control and escape in mice.EMBO J. 2013 Sep 11;32(18):2454-62. doi: 10.1038/emboj.2013.163. Epub 2013 Jul 19. EMBO J. 2013. PMID: 23872947 Free PMC article.
References
-
- Arthur LO, Bess JW, Snowder RC, II, Benveniste RE, Mann DL, Chermann J-C, Henderson LE. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–1938. - PubMed
-
- Balcaitis S, Weinstein JR, Li S, Chamberlain JS, Moller T. Lentiviral transduction of microglial cells. Glia. 2005;50:48–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

