TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure
- PMID: 19617407
- PMCID: PMC2755979
- DOI: 10.1152/ajpheart.00241.2009
TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure
Abstract
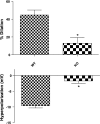
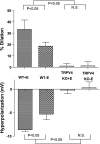
Transient receptor potential vanilloid 4 (TRPV4) channels have been implicated as mediators of calcium influx in both endothelial and vascular smooth muscle cells and are potentially important modulators of vascular tone. However, very little is known about the functional roles of TRPV4 in the resistance vasculature or how these channels influence hemodynamic properties. In the present study, we examined arterial vasomotor activity in vitro and recorded blood pressure dynamics in vivo using TRPV4 knockout (KO) mice. Acetylcholine-induced hyperpolarization and vasodilation were reduced by approximately 75% in mesenteric resistance arteries from TRPV4 KO versus wild-type (WT) mice. Furthermore, 11,12-epoxyeicosatrienoic acid (EET), a putative endothelium-derived hyperpolarizing factor, activated a TRPV4-like cation current and hyperpolarized the membrane of vascular smooth muscle cells, resulting in the dilation of mesenteric arteries from WT mice. In contrast, 11,12-EET had no effect on membrane potential, diameter, or ionic currents in the mesenteric arteries from TRPV4 KO mice. A disruption of the endothelium reduced 11,12-EET-induced hyperpolarization and vasodilatation by approximately 50%. A similar inhibition of these responses was observed following the block of endothelial (small and intermediate conductance) or smooth muscle (large conductance) K(+) channels, suggesting a link between 11,12-EET activity, TRPV4, and K(+) channels in endothelial and smooth muscle cells. Finally, we found that hypertension induced by the inhibition of nitric oxide synthase was greater in TRPV4 KO compared with WT mice. These results support the conclusion that both endothelial and smooth muscle TRPV4 channels are critically involved in the vasodilation of mesenteric arteries in response to endothelial-derived factors and suggest that in vivo this mechanism opposes the effects of hypertensive stimuli.
Figures



References
-
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996. - PubMed
-
- Coleman HA, Tare M, Parkington HC. EDHF is not K+ but may be due to spread of current from the endothelium in guinea pig arterioles. Am J Physiol Heart Circ Physiol 280: H2478–H2483, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

