Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion
- PMID: 19617536
- PMCID: PMC2718387
- DOI: 10.1073/pnas.0804273106
Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion
Abstract
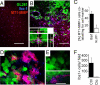
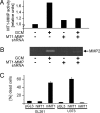
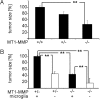
Diffuse infiltration of glioma cells into normal brain tissue is considered to be a main reason for the unfavorable outcomes of patients with malignant gliomas. Invasion of glioma cells into the brain parenchyma is facilitated by metalloprotease-mediated degradation of the extracellular matrix. Metalloproteases are released as inactive pro-forms and get activated upon cleavage by membrane bound metalloproteases. Here, we show that membrane type 1 metalloprotease (MT1-MMP) is up-regulated in glioma-associated microglia, but not in the glioma cells. Overexpression of MT1-MMP is even lethal for glioma cells. Glioma-released factors trigger the expression and activity of MT1-MMP via microglial toll-like receptors and the p38 MAPK pathway, as deletion of the toll-like receptor adapter protein MyD88 or p38 inhibition prevented MT1-MMP expression and activity in cultured microglial cells. Microglial MT1-MMP in turn activates glioma-derived pro-MMP-2 and promotes glioma expansion, as shown in an ex vivo model using MT1-MMP-deficient brain tissue and a microglia depletion paradigm. Finally, MyD88 deficiency or microglia depletion largely attenuated glioma expansion in 2 independent in vivo models.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP) J Cell Physiol. 2004;200:2–10. - PubMed
-
- Mayes DA, et al. PAX6 suppresses the invasiveness of glioblastoma cells and the expression of the matrix metalloproteinase-2 gene. Cancer Res. 2006;66:9809–9817. - PubMed
-
- Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. - PubMed
-
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

