Anti-VEGF single-chain antibody GLAF-1 encoded by oncolytic vaccinia virus significantly enhances antitumor therapy
- PMID: 19617539
- PMCID: PMC2722284
- DOI: 10.1073/pnas.0900660106
Anti-VEGF single-chain antibody GLAF-1 encoded by oncolytic vaccinia virus significantly enhances antitumor therapy
Abstract
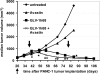
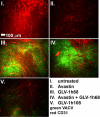
We previously reported that the replication-competent vaccinia virus (VACV) GLV-1h68 shows remarkable oncolytic activity and efficacy in different animal models as a single treatment modality and also in combination with chemotherapy [Yu YA, et al. (2009) Mol Cancer Ther 8:141-151]. Here, we report the construction of 3 VACV strains encoding GLAF-1, a previously undescribed engineered single-chain antibody (scAb). This unique scAb is transcribed from 3 vaccinia promoters (synthetic early, early/late, and late) and directed against both human and murine VEGFs. The expression of GLAF-1 was demonstrated in cell cultures. Also, the replication efficiency of all GLAF-1-expressing VACV strains in cell culture was similar to that of the parental GLV-1h68 virus. Successful tumor-specific delivery and continued production of functional scAb derived from individual VACV strains were obtained in tumor xenografts following a single intravenous injection of the virus. The VACV strains expressing the scAb exhibited significantly enhanced therapeutic efficacy in comparison to treatment of human tumor xenografts with the parental virus GLV-1h68. This enhanced efficacy was comparable to the concomitant treatment of tumors with a one-time i.v. injection of GLV-1h68 and multiple i.p. injections of Avastin. Taken together, the VACV-mediated delivery and production of immunotherapeutic anti-VEGF scAb in colonized tumors may open the way for a unique therapy concept: tumor-specific, locally amplified drug therapy in humans.
Conflict of interest statement
Conflict of interest statement: A.F., Y.A.Y., N.C., Q.Z., and A.A.S. are employees of Genelux Corporation.
Figures


Similar articles
-
Virotherapy of canine tumors with oncolytic vaccinia virus GLV-1h109 expressing an anti-VEGF single-chain antibody.PLoS One. 2012;7(10):e47472. doi: 10.1371/journal.pone.0047472. Epub 2012 Oct 16. PLoS One. 2012. PMID: 23091626 Free PMC article.
-
Growth inhibition of different human colorectal cancer xenografts after a single intravenous injection of oncolytic vaccinia virus GLV-1h68.J Transl Med. 2013 Mar 26;11:79. doi: 10.1186/1479-5876-11-79. J Transl Med. 2013. PMID: 23531320 Free PMC article.
-
Evaluation of a new recombinant oncolytic vaccinia virus strain GLV-5b451 for feline mammary carcinoma therapy.PLoS One. 2014 Aug 5;9(8):e104337. doi: 10.1371/journal.pone.0104337. eCollection 2014. PLoS One. 2014. PMID: 25093734 Free PMC article.
-
Immunotherapeutic potential of oncolytic vaccinia virus.Immunol Res. 2011 Aug;50(2-3):286-93. doi: 10.1007/s12026-011-8211-4. Immunol Res. 2011. PMID: 21717084 Review.
-
The employment of vaccinia virus for colorectal cancer treatment: A review of preclinical and clinical studies.Hum Vaccin Immunother. 2022 Nov 30;18(6):2143698. doi: 10.1080/21645515.2022.2143698. Epub 2022 Nov 11. Hum Vaccin Immunother. 2022. PMID: 36369829 Free PMC article. Review.
Cited by
-
Vaccinia virus-mediated melanin production allows MR and optoacoustic deep tissue imaging and laser-induced thermotherapy of cancer.Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3316-20. doi: 10.1073/pnas.1216916110. Epub 2013 Feb 11. Proc Natl Acad Sci U S A. 2013. PMID: 23401518 Free PMC article.
-
Virotherapy of canine tumors with oncolytic vaccinia virus GLV-1h109 expressing an anti-VEGF single-chain antibody.PLoS One. 2012;7(10):e47472. doi: 10.1371/journal.pone.0047472. Epub 2012 Oct 16. PLoS One. 2012. PMID: 23091626 Free PMC article.
-
Growth inhibition of different human colorectal cancer xenografts after a single intravenous injection of oncolytic vaccinia virus GLV-1h68.J Transl Med. 2013 Mar 26;11:79. doi: 10.1186/1479-5876-11-79. J Transl Med. 2013. PMID: 23531320 Free PMC article.
-
Functional hyper-IL-6 from vaccinia virus-colonized tumors triggers platelet formation and helps to alleviate toxicity of mitomycin C enhanced virus therapy.J Transl Med. 2012 Jan 11;10:9. doi: 10.1186/1479-5876-10-9. J Transl Med. 2012. PMID: 22236378 Free PMC article.
-
Oncolytic vaccinia virus and cancer immunotherapy.Front Immunol. 2024 Jan 12;14:1324744. doi: 10.3389/fimmu.2023.1324744. eCollection 2023. Front Immunol. 2024. PMID: 38283361 Free PMC article. Review.
References
-
- American Cancer Society. Cancer Facts and Figures 2008. Atlanta: American Cancer Society; 2008.
-
- Jain RK. Delivery of novel therapeutic agents in tumors: Physiological barriers and strategies. J Natl Cancer Inst. 1989;81:570–576. - PubMed
-
- Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Delivery Rev. 2001;46:149–168. - PubMed
-
- Reilly RM, et al. Problems of delivery of monoclonal antibodies. Pharmaceutical and pharmacokinetic solutions. Clin Pharmacokinet. 1995;28:126–142. - PubMed
-
- Manegold C. Bevacizumab for the treatment of advanced non-small-cell lung cancer. Expert Rev Anticancer Ther. 2008;8:689–699. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

