Inactivating germ-line and somatic mutations in polypeptide N-acetylgalactosaminyltransferase 12 in human colon cancers
- PMID: 19617566
- PMCID: PMC2722285
- DOI: 10.1073/pnas.0901454106
Inactivating germ-line and somatic mutations in polypeptide N-acetylgalactosaminyltransferase 12 in human colon cancers
Abstract
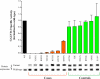
Aberrant glycosylation is a pathological alteration that is widespread in colon cancer, and usually accompanies the onset and progression of the disease. To date, the molecular mechanisms underlying aberrant glycosylation remain largely unknown. In this study, we identify somatic and germ-line mutations in the gene encoding for polypeptide N-acetylgalactosaminyltransferase 12 (GALNT12) in individuals with colon cancer. Biochemical analyses demonstrate that each of the 8 GALNT12 mutations identified inactivates the normal function of the GALNT enzyme in initiating mucin type O-linked protein glycosylation. Two of these inactivating GALNT12 mutations were identified as acquired somatic mutations in a set of 30 microsatellite stable colon tumors. Relative to background gene mutation rates, finding these somatic GALNT12 mutations was statistically significant at P < 0.001. Six additional inactivating GALNT12 mutations were detected as germ-line changes carried by patients with colon cancer; however, no inactivating variants were detected among cancer-free controls (P = 0.005). Notably, in 3 of the 6 individuals harboring inactivating germ-line GALNT12 mutations, both a colon cancer and a second independent epithelial cancer had developed. These findings suggest that genetic defects in the O-glycosylation pathway in part underlie aberrant glycosylation in colon cancers, and they contribute to the development of a subset of these malignancies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochim Biophys Acta. 1999;1473:67–95. - PubMed
-
- Ten Hagen KG, Fritz TA, Tabak LA. All in the family: The UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology. 2003;13:1R–16R. - PubMed
-
- Wood LD, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. - PubMed
-
- Guo JM, et al. Molecular cloning and characterization of a novel member of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family, pp-GalNAc-T12. FEBS Lett. 2002;524:211–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA078834/CA/NCI NIH HHS/United States
- CA121113/CA/NCI NIH HHS/United States
- R01 CA130901/CA/NCI NIH HHS/United States
- R01 CA121113/CA/NCI NIH HHS/United States
- CA 43460/CA/NCI NIH HHS/United States
- P30 CA043703/CA/NCI NIH HHS/United States
- U54 CA116867/CA/NCI NIH HHS/United States
- CA116867/CA/NCI NIH HHS/United States
- CA78834/CA/NCI NIH HHS/United States
- R37 CA057345/CA/NCI NIH HHS/United States
- R01 CA057345/CA/NCI NIH HHS/United States
- P30 CA43703/CA/NCI NIH HHS/United States
- CA57345/CA/NCI NIH HHS/United States
- R37 CA043460/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P30 DK027651/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

