Fibronectin binds and enhances the activity of bone morphogenetic protein 1
- PMID: 19617627
- PMCID: PMC2757989
- DOI: 10.1074/jbc.M109.024125
Fibronectin binds and enhances the activity of bone morphogenetic protein 1
Abstract
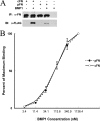
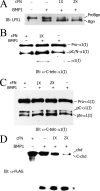
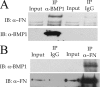
Bone morphogenetic protein-1-like proteinases play key roles in formation of the extracellular matrix (ECM) in vertebrates via biosynthetic processing of precursors into mature functional proteins involved in ECM assembly. Such processing includes proteolytic activation of the zymogen for lysyl oxidase. Fibronectin (FN) is an abundant protein component of the ECM that is capable of regulating manifold cellular functions through its interactions with various ECM and cell surface proteins. It was previously shown that proteolytic activation of lysyl oxidase is much reduced in cultures of FN-null mouse embryo fibroblasts (MEFs). Here we demonstrate that cellular fibronectin, the form produced by fibroblasts and various other tissue cell types, and plasma fibronectin bind BMP1 with dissociation constants (KD) of approximately 100 nM, consistent with a physiological role. Also consistent with such a role, cellular fibronectin FN is shown to positively regulate BMP1 processing activity against Chordin, probiglycan, and type I procollagen in vitro. Endogenous FN and BMP1 are demonstrated to co-localize in cell layers and to form complexes in culture medium. In addition, processing of endogenous BMP1 substrates Chordin, probiglycan, and procollagen is demonstrated to be strikingly reduced in cultures of FN(-/-) MEFs compared with FN(+/-) MEF cultures despite similar levels of endogenous BMP1. These data support the conclusion that FN binds BMP1-like proteinases in vivo and that FN is an important determinant of the in vivo activity levels of BMP1-like proteinases.
Figures





Similar articles
-
Fibronectin matrix as a scaffold for procollagen proteinase binding and collagen processing.Mol Biol Cell. 2019 Aug 1;30(17):2218-2226. doi: 10.1091/mbc.E19-03-0140. Epub 2019 Jun 26. Mol Biol Cell. 2019. PMID: 31242089 Free PMC article.
-
Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures.J Biol Chem. 2001 Jun 22;276(25):22537-43. doi: 10.1074/jbc.M102352200. Epub 2001 Apr 19. J Biol Chem. 2001. PMID: 11313359
-
Bone morphogenetic protein-1 processes probiglycan.J Biol Chem. 2000 Sep 29;275(39):30504-11. doi: 10.1074/jbc.M004846200. J Biol Chem. 2000. PMID: 10896944
-
Developmental roles of the BMP1/TLD metalloproteinases.Birth Defects Res C Embryo Today. 2006 Mar;78(1):47-68. doi: 10.1002/bdrc.20060. Birth Defects Res C Embryo Today. 2006. PMID: 16622848 Review.
-
Fibronectin: Molecular Structure, Fibrillar Structure and Mechanochemical Signaling.Cells. 2021 Sep 16;10(9):2443. doi: 10.3390/cells10092443. Cells. 2021. PMID: 34572092 Free PMC article. Review.
Cited by
-
Fibronectin matrix as a scaffold for procollagen proteinase binding and collagen processing.Mol Biol Cell. 2019 Aug 1;30(17):2218-2226. doi: 10.1091/mbc.E19-03-0140. Epub 2019 Jun 26. Mol Biol Cell. 2019. PMID: 31242089 Free PMC article.
-
Agonists and Antagonists of TGF-β Family Ligands.Cold Spring Harb Perspect Biol. 2016 Aug 1;8(8):a021923. doi: 10.1101/cshperspect.a021923. Cold Spring Harb Perspect Biol. 2016. PMID: 27413100 Free PMC article. Review.
-
First evidence of bone morphogenetic protein 1 expression and activity in sheep ovarian follicles.Biol Reprod. 2010 Jul;83(1):138-46. doi: 10.1095/biolreprod.109.082115. Epub 2010 Mar 31. Biol Reprod. 2010. PMID: 20357269 Free PMC article.
-
Molecular Mechanism Responsible for Fibronectin-controlled Alterations in Matrix Stiffness in Advanced Chronic Liver Fibrogenesis.J Biol Chem. 2016 Jan 1;291(1):72-88. doi: 10.1074/jbc.M115.691519. Epub 2015 Nov 9. J Biol Chem. 2016. PMID: 26553870 Free PMC article.
-
Carrier systems for bone morphogenetic proteins: An overview of biomaterials used for dentoalveolar and maxillofacial bone regeneration.Jpn Dent Sci Rev. 2022 Nov;58:316-327. doi: 10.1016/j.jdsr.2022.10.001. Epub 2022 Oct 19. Jpn Dent Sci Rev. 2022. PMID: 36281233 Free PMC article. Review.
References
-
- Pankov R., Yamada K. M. (2002) J. Cell Sci. 115, 3861–3863 - PubMed
-
- Hynes R. O. (1990) Fibronectins, Springer-Verlag New York Inc., New York
-
- Magnusson M. K., Mosher D. F. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 1363–1370 - PubMed
-
- Mao Y., Schwarzbauer J. E. (2005) Matrix Biol. 24, 389–399 - PubMed
-
- Ffrench-Constant C. (1995) Exp. Cell Res. 221, 261–271 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

