Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10
- PMID: 19617629
- PMCID: PMC2757958
- DOI: 10.1074/jbc.M109.019786
Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10
Abstract
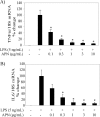
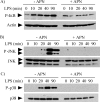
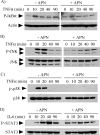
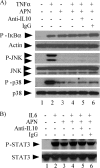
Macrophages participate pivotally in the pathogenesis of many chronic inflammatory diseases including atherosclerosis. Adiponectin, a vasculoprotective molecule with insulin-sensitizing and anti-atherogenic properties, suppresses pro-inflammatory gene expression in macrophages by mechanisms that remain incompletely understood. This study investigated the effects of adiponectin on major pro-inflammatory signaling pathways in human macrophages. We demonstrate that pretreatment of these cells with adiponectin inhibits phosphorylation of nuclear factor kappaB inhibitor (IkappaB), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK), induced by either lipopolysaccharide (LPS) or tumor necrosis factor (TNF) alpha, as well as STAT3 phosphorylation induced by interleukin-6 (IL6). Antagonism of IL10 by either neutralizing antibodies or siRNA-mediated silencing did not abrogate the anti-inflammatory actions of adiponectin, indicating that the ability of adiponectin to render human macrophages tolerant to various pro-inflammatory stimuli does not require this cytokine. A systematic search for adiponectin-inducible genes with established anti-inflammatory properties revealed that adiponectin augmented the expression of A20, suppressor of cytokine signaling (SOCS) 3, B-cell CLL/lymphoma (BCL) 3, TNF receptor-associated factor (TRAF) 1, and TNFAIP3-interacting protein (TNIP) 3. These results suggest that adiponectin triggers a multifaceted response in human macrophages by inducing the expression of various anti-inflammatory proteins that act at different levels in concert to suppress macrophage activation.
Figures
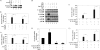
References
-
- Rocha V. Z., Libby P. (2009) Nat. Rev. Cardiol. 6, 399–409 - PubMed
-
- Scherer P. E. (2006) Diabetes 55, 1537–1545 - PubMed
-
- Okamoto Y., Kihara S., Funahashi T., Matsuzawa Y., Libby P. (2006) Clin. Sci. 110, 267–278 - PubMed
-
- Zhu W., Cheng K. K., Vanhoutte P. M., Lam K. S., Xu A. (2008) Clin. Sci. 114, 361–374 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

