Neutralization of granulocyte macrophage colony-stimulating factor decreases amyloid beta 1-42 and suppresses microglial activity in a transgenic mouse model of Alzheimer's disease
- PMID: 19617638
- PMCID: PMC2748895
- DOI: 10.1093/hmg/ddp331
Neutralization of granulocyte macrophage colony-stimulating factor decreases amyloid beta 1-42 and suppresses microglial activity in a transgenic mouse model of Alzheimer's disease
Abstract
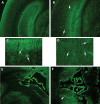
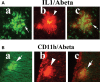
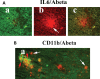
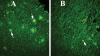
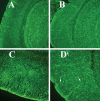
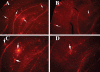
The purpose of our study was to investigate microglia and astrocytes that are associated with human mutant amyloid precursor protein and amyloid beta (Abeta). We investigated whether the anti-granulocyte-macrophage-colony stimulating factor (GM-CSF) antibody can suppress microglial activity and decrease Abeta production in Alzheimer's disease transgenic mice (Tg2576 line). An antibody to mouse GM-CSF was introduced by intracerebroventricular (ICV) injections into the brains of 10-month-old Tg2576 male mice. We assessed the effect of several GM-CSF-associated cytokines on microglial activities and their association with Abeta using quantitative real-time RT-PCR, immunoblotting, immunohistochemistry analyses in anti-GM-CSF antibody-injected Tg2576 mice. Using sandwich ELISA technique, we measured intraneuronal Abeta in Tg2576 mice injected with GM-CSF antibody and PBS vehicle-injected control Tg2576 mice. Using double-labeling immunofluorescence analysis of intraneuronal Abeta, Abeta deposits and pro-inflammatory cytokines, we assessed the relationship between Abeta deposits and microglial markers in the Tg2576 mice, and also in the anti-GM-CSF antibody-injected Tg2576 mice. Our real-time RT-PCR analysis showed an increase in the mRNA expression of IL6, CD11c, IL1beta, CD40 and CD11b in the cerebral cortices of the Tg2576 mice compared with their littermate non-transgenic controls. Immunohistochemistry findings of microglial markers agreed with our real-time RT-PCR results. Interestingly, we found significantly decreased levels of activated microglia and Abeta deposits in anti-GM-CSF antibody-injected Tg2576 mice compared with PBS vehicle-injected Tg2576 mice. Findings from our real-time RT-PCR and immunoblotting analysis agreed with immunohistochemistry results. Our double-labeling analyses of intraneuronal Abeta and CD40 revealed that intraneuronal Abeta is associated with neuronal expression of CD40 in Tg2576 mice. Our quantitative sandwich ELISA analysis revealed decreased levels of soluble Abeta1-42 and increased levels of Abeta1-40 in Tg2576 mice injected with the anti-GM-CSF antibody, suggesting that anti-GM-CSF antibody alone decreases soluble Abeta1-42 production and suppresses microglial activity in Tg2576 mice. These findings indicating the ability of the anti-GM-CSF antibody to reduce Abeta1-42 and microglial activity in Tg2576 mice may have therapeutic implications for Alzheimer's disease.
Figures





Similar articles
-
Granulocyte-macrophage colony-stimulating factor antibody suppresses microglial activity: implications for anti-inflammatory effects in Alzheimer's disease and multiple sclerosis.J Neurochem. 2009 Dec;111(6):1514-28. doi: 10.1111/j.1471-4159.2009.06432.x. Epub 2009 Oct 14. J Neurochem. 2009. PMID: 19840215 Free PMC article.
-
Immunization of Alzheimer model mice with adenovirus vectors encoding amyloid beta-protein and GM-CSF reduces amyloid load in the brain.Neurosci Lett. 2004 Nov 11;370(2-3):218-23. doi: 10.1016/j.neulet.2004.08.059. Neurosci Lett. 2004. PMID: 15488326
-
Overexpression of human S100B exacerbates cerebral amyloidosis and gliosis in the Tg2576 mouse model of Alzheimer's disease.Glia. 2010 Feb;58(3):300-14. doi: 10.1002/glia.20924. Glia. 2010. PMID: 19705461 Free PMC article.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
Should We Open Fire on Microglia? Depletion Models as Tools to Elucidate Microglial Role in Health and Alzheimer's Disease.Int J Mol Sci. 2021 Sep 8;22(18):9734. doi: 10.3390/ijms22189734. Int J Mol Sci. 2021. PMID: 34575898 Free PMC article. Review.
Cited by
-
Peripheral sTREM2-Related Inflammatory Activity Alterations in Early-Stage Alzheimer's Disease.J Immunol. 2022 May 15;208(10):2283-2299. doi: 10.4049/jimmunol.2100771. Epub 2022 May 6. J Immunol. 2022. PMID: 35523454 Free PMC article.
-
GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia.J Neuroinflammation. 2012 Dec 13;9:268. doi: 10.1186/1742-2094-9-268. J Neuroinflammation. 2012. PMID: 23234315 Free PMC article.
-
MitoQ, a mitochondria-targeted antioxidant, delays disease progression and alleviates pathogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis.Biochim Biophys Acta. 2013 Dec;1832(12):2322-31. doi: 10.1016/j.bbadis.2013.09.005. Epub 2013 Sep 19. Biochim Biophys Acta. 2013. PMID: 24055980 Free PMC article.
-
Mitochondrial DNA deletions and differential mitochondrial DNA content in Rhesus monkeys: implications for aging.Biochim Biophys Acta. 2012 Feb;1822(2):111-9. doi: 10.1016/j.bbadis.2011.10.014. Epub 2011 Oct 28. Biochim Biophys Acta. 2012. PMID: 22056405 Free PMC article.
-
Mitochondria-targeted catalase reduces abnormal APP processing, amyloid β production and BACE1 in a mouse model of Alzheimer's disease: implications for neuroprotection and lifespan extension.Hum Mol Genet. 2012 Jul 1;21(13):2973-90. doi: 10.1093/hmg/dds128. Epub 2012 Apr 5. Hum Mol Genet. 2012. PMID: 22492996 Free PMC article.
References
-
- Selkoe D.J. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. - PubMed
-
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. 2006;12:1005–1015. - PubMed
-
- LaFerla F.M., Green K.N., Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat. Rev. Neurosci. 2007;8:499–509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

