Virologic and immunologic correlates with the magnitude of antibody responses to the hepatitis A vaccine in HIV-infected children on highly active antiretroviral treatment
- PMID: 19617848
- PMCID: PMC2836885
- DOI: 10.1097/QAI.0b013e3181b011f6
Virologic and immunologic correlates with the magnitude of antibody responses to the hepatitis A vaccine in HIV-infected children on highly active antiretroviral treatment
Abstract
Background: HIV-infected individuals mount poor antibody responses to vaccines. We sought to identify the immunologic and virologic factors associated with a robust response to hepatitis A virus (HAV) vaccine in children on highly active antiretroviral treatment.
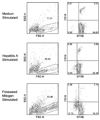
Methods: One hundred fifty-two pediatric highly active antiretroviral treatment recipients immunized against HAV at weeks 0 and 24 had anti-HAV antibodies, CD4+, CD8+, and CD19+ cell percent assessed at weeks 0 and 32. Subgroups had HIV viremia, B- and T-cell subpopulations, and cell-mediated immunity (CMI) to HAV and other stimulants measured.
Results: Anti-HAV antibodies after complete vaccination correlated positively with CD4+ percent and CD19+ percent and negatively with viremia and CD8+ percent at baseline, but not at 32 weeks. There were no significant correlations between anti-HAV antibodies and B- or T-cell-naïve, memory, or activated subpopulations or non-HAV CMI. Compared with children who remained HAV-CMI-negative, those who mounted HAV-CMI in response to vaccination had higher anti-HAV antibody titers and CD19+ CD21+ CD27+ memory B cell percent at 32 weeks, but no other differences.
Conclusions: In HIV-infected children on highly active antiretroviral treatment, control of viral replication and conserved or reconstituted CD19+ and CD4+ cell numbers and function determine a robust antibody response to anti-HAV primary immunization. Our data support a bidirectional B- and T-cell cooperation in the response to the HAV vaccine.
Conflict of interest statement
The authors have no conflicts of interest.
Figures
References
-
- Abzug MJ, Pelton SI, Song LY, et al. Immunogenicity, safety, and predictors of response after a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2006;25:920–929. - PubMed
-
- Hess G, Clemens R, Bienzle U, et al. Immunogenicity and safety of an inactivated hepatitis A vaccine in anti-HIV positive and negative homosexual men. J Med Virol. 1995;46:40–42. - PubMed
-
- Kemper CA, Haubrich R, Frank I, et al. Safety and immunogenicity of hepatitis A vaccine in human immunodeficiency virus-infected patients: a double-blind, randomized, placebo-controlled trial. J Infect Dis. 2003;187:1327–1331. - PubMed
-
- Mount AM, Mwapasa V, Elliott SR, et al. Impairment of humoral immunity to Plasmodium falciparum malaria in pregnancy by HIV infection. Lancet. 2004;363:1860–1867. - PubMed
-
- Neilsen GA, Bodsworth NJ, Watts N. Response to hepatitis A vaccination in human immunodeficiency virus-infected and -uninfected homosexual men. J Infect Dis. 1997;176:1064–1067. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials