Remission of invasive, cancer stem-like glioblastoma xenografts using lentiviral vector-mediated suicide gene therapy
- PMID: 19617915
- PMCID: PMC2707627
- DOI: 10.1371/journal.pone.0006314
Remission of invasive, cancer stem-like glioblastoma xenografts using lentiviral vector-mediated suicide gene therapy
Abstract
Background: Glioblastoma is the most frequent and most malignant primary brain tumor with a poor prognosis. The translation of therapeutic strategies for glioblastoma from the experimental phase into the clinic has been limited by insufficient animal models, which lack important features of human tumors. Lentiviral gene therapy is an attractive therapeutic option for human glioblastoma, which we validated in a clinically relevant animal model.
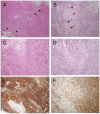
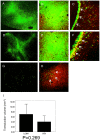
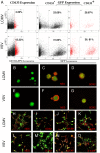
Methodology/principal findings: We used a rodent xenograft model that recapitulates the invasive and angiogenic features of human glioblastoma to analyze the transduction pattern and therapeutic efficacy of lentiviral pseudotyped vectors. Both, lymphocytic choriomeningitis virus glycoprotein (LCMV-GP) and vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped lentiviral vectors very efficiently transduced human glioblastoma cells in vitro and in vivo. In contrast, pseudotyped gammaretroviral vectors, similar to those evaluated for clinical therapy of glioblastoma, showed inefficient gene transfer in vitro and in vivo. Both pseudotyped lentiviral vectors transduced cancer stem-like cells characterized by their CD133-, nestin- and SOX2-expression, the ability to form spheroids in neural stem cell medium and to express astrocytic and neuronal differentiation markers under serum conditions. In a therapeutic approach using the suicide gene herpes simplex virus thymidine kinase (HSV-1-tk) fused to eGFP, both lentiviral vectors mediated a complete remission of solid tumors as seen on MRI resulting in a highly significant survival benefit (p<0.001) compared to control groups. In all recurrent tumors, surviving eGFP-positive tumor cells were found, advocating prodrug application for several cycles to even enhance and prolong the therapeutic effect.
Conclusions/significance: In conclusion, lentiviral pseudotyped vectors are promising candidates for gene therapy of glioma in patients. The inefficient gene delivery by gammaretroviral vectors is in line with the results obtained in clinical therapy for GBM and thus confirms the high reproducibility of the invasive glioma animal model for translational research.
Conflict of interest statement
Figures




References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Mahesparan R, Read TA, Lund-Johansen M, Skaftnesmo KO, Bjerkvig R, et al. Expression of extracellular matrix components in a highly infiltrative in vivo glioma model. Acta Neuropathol. 2003;105:49–57. - PubMed
-
- Huszthy PC, Goplen D, Thorsen F, Immervoll H, Wang J, et al. Oncolytic herpes simplex virus type-1 therapy in a highly infiltrative animal model of human glioblastoma. Clin Cancer Res. 2008;14:1571–1580. - PubMed
-
- Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

