Effects of osmolytes on the SLN1-YPD1-SSK1 phosphorelay system from Saccharomyces cerevisiae
- PMID: 19618914
- PMCID: PMC2753885
- DOI: 10.1021/bi900886g
Effects of osmolytes on the SLN1-YPD1-SSK1 phosphorelay system from Saccharomyces cerevisiae
Abstract
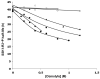
The multistep His-Asp phosphorelay system in Saccharomyces cerevisiae allows cells to adapt to osmotic, oxidative, and other environmental stresses. The pathway consists of a hybrid histidine kinase SLN1, a histidine-containing phosphotransfer (HPt) protein YPD1, and two response regulator proteins, SSK1 and SKN7. Under nonosmotic stress conditions, the SLN1 sensor kinase is active, and phosphoryl groups are shuttled through YPD1 to SSK1, therefore maintaining the response regulator protein in a constitutively phosphorylated state. The cellular response to hyperosmotic stress involves rapid efflux of water and changes in intracellular ion and osmolyte concentration. In this study, we examined the individual and combined effects of NaCl and glycerol on phosphotransfer rates within the SLN1-YPD1-SSK1 phosphorelay. The results show that the combined effects of glycerol and NaCl on the phosphotransfer reaction rates are different from the individual effects of glycerol and NaCl. The combinatory effect is likely more representative of the in vivo changes that occur during hyperosmotic stress. In addition, the effect of osmolyte concentration on the half-life of the phosphorylated SSK1 receiver domain in the presence/absence of YPD1 was evaluated. Our findings demonstrate that increasing osmolyte concentrations negatively affect the YPD1 x SSK1-P interaction, thereby facilitating dephosphorylation of SSK1 and activating the HOG1 MAP kinase cascade. In contrast, at the highest osmolyte concentrations, reflective of the osmoadaptation phase of the signaling pathway, the kinetics of the phosphorelay favor production of SSK1-P and inhibition of the HOG1 pathway.
Figures


Similar articles
-
Insights revealed by the co-crystal structure of the Saccharomyces cerevisiae histidine phosphotransfer protein Ypd1 and the receiver domain of its downstream response regulator Ssk1.Protein Sci. 2019 Dec;28(12):2099-2111. doi: 10.1002/pro.3755. Epub 2019 Nov 7. Protein Sci. 2019. PMID: 31642125 Free PMC article.
-
Kinetic analysis of YPD1-dependent phosphotransfer reactions in the yeast osmoregulatory phosphorelay system.Biochemistry. 2005 Jan 11;44(1):377-86. doi: 10.1021/bi048433s. Biochemistry. 2005. PMID: 15628880
-
Robust network structure of the Sln1-Ypd1-Ssk1 three-component phospho-relay prevents unintended activation of the HOG MAPK pathway in Saccharomyces cerevisiae.BMC Syst Biol. 2015 Mar 25;9:17. doi: 10.1186/s12918-015-0158-y. BMC Syst Biol. 2015. PMID: 25888817 Free PMC article.
-
Histidine phosphotransfer proteins in fungal two-component signal transduction pathways.Eukaryot Cell. 2013 Aug;12(8):1052-60. doi: 10.1128/EC.00083-13. Epub 2013 Jun 14. Eukaryot Cell. 2013. PMID: 23771905 Free PMC article. Review.
-
Yeast go the whole HOG for the hyperosmotic response.Trends Genet. 2002 Aug;18(8):405-12. doi: 10.1016/s0168-9525(02)02723-3. Trends Genet. 2002. PMID: 12142009 Review.
Cited by
-
Regulatory role of glycerol in Candida albicans biofilm formation.mBio. 2013 Apr 9;4(2):e00637-12. doi: 10.1128/mBio.00637-12. mBio. 2013. PMID: 23572557 Free PMC article.
-
The phosphotransferase VanU represses expression of four qrr genes antagonizing VanO-mediated quorum-sensing regulation in Vibrio anguillarum.Microbiology (Reading). 2011 Dec;157(Pt 12):3324-3339. doi: 10.1099/mic.0.051011-0. Epub 2011 Sep 21. Microbiology (Reading). 2011. PMID: 21948044 Free PMC article.
-
Stress signalling to fungal stress-activated protein kinase pathways.FEMS Microbiol Lett. 2010 May;306(1):1-8. doi: 10.1111/j.1574-6968.2010.01937.x. Epub 2010 Feb 24. FEMS Microbiol Lett. 2010. PMID: 20345377 Free PMC article. Review.
-
Antioxidant Systems of Plant Pathogenic Fungi: Functions in Oxidative Stress Response and Their Regulatory Mechanisms.Plant Pathol J. 2024 Jun;40(3):235-250. doi: 10.5423/PPJ.RW.01.2024.0001. Epub 2024 Jun 1. Plant Pathol J. 2024. PMID: 38835295 Free PMC article. Review.
-
Use of restrained molecular dynamics to predict the conformations of phosphorylated receiver domains in two-component signaling systems.Proteins. 2017 Jan;85(1):155-176. doi: 10.1002/prot.25207. Epub 2016 Nov 20. Proteins. 2017. PMID: 27802580 Free PMC article.
References
-
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu. Rev. Biochem. 2000;69:183–215. - PubMed
-
- West AH, Stock AM. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 2001;26:369–376. - PubMed
-
- Appleby JL, Parkinson JS, Bourret RB. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. - PubMed
-
- Morgan BA, Bouquin N, Johnston LH. Two-component signal-transduction systems in budding yeast MAP a different pathway? Trends Cell Biol. 1995;5:453–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

