Nitroxyl radical plus hydroxylamine pseudo self-exchange reactions: tunneling in hydrogen atom transfer
- PMID: 19618933
- PMCID: PMC2775461
- DOI: 10.1021/ja904400d
Nitroxyl radical plus hydroxylamine pseudo self-exchange reactions: tunneling in hydrogen atom transfer
Abstract
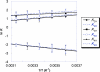
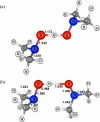
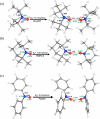
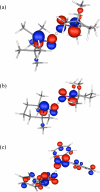
Bimolecular rate constants have been measured for reactions that involve hydrogen atom transfer (HAT) from hydroxylamines to nitroxyl radicals, using the stable radicals TEMPO(*) (2,2,6,6-tetramethylpiperidine-1-oxyl radical), 4-oxo-TEMPO(*) (2,2,6,6-tetramethyl-4-oxo-piperidine-1-oxyl radical), di-tert-butylnitroxyl ((t)Bu(2)NO(*)), and the hydroxylamines TEMPO-H, 4-oxo-TEMPO-H, 4-MeO-TEMPO-H (2,2,6,6-tetramethyl-N-hydroxy-4-methoxy-piperidine), and (t)Bu(2)NOH. The reactions have been monitored by UV-vis stopped-flow methods, using the different optical spectra of the nitroxyl radicals. The HAT reactions all have |DeltaG (o)| < or = 1.4 kcal mol(-1) and therefore are close to self-exchange reactions. The reaction of 4-oxo-TEMPO(*) + TEMPO-H --> 4-oxo-TEMPO-H + TEMPO(*) occurs with k(2H,MeCN) = 10 +/- 1 M(-1) s(-1) in MeCN at 298 K (K(2H,MeCN) = 4.5 +/- 1.8). Surprisingly, the rate constant for the analogous deuterium atom transfer reaction is much slower: k(2D,MeCN) = 0.44 +/- 0.05 M(-1) s(-1) with k(2H,MeCN)/k(2D,MeCN) = 23 +/- 3 at 298 K. The same large kinetic isotope effect (KIE) is found in CH(2)Cl(2), 23 +/- 4, suggesting that the large KIE is not caused by solvent dynamics or hydrogen bonding to solvent. The related reaction of 4-oxo-TEMPO(*) with 4-MeO-TEMPO-H(D) also has a large KIE, k(3H)/k(3D) = 21 +/- 3 in MeCN. For these three reactions, the E(aD) - E(aH) values, between 0.3 +/- 0.6 and 1.3 +/- 0.6 kcal mol(-1), and the log(A(H)/A(D)) values, between 0.5 +/- 0.7 and 1.1 +/- 0.6, indicate that hydrogen tunneling plays an important role. The related reaction of (t)Bu(2)NO(*) + TEMPO-H(D) in MeCN has a large KIE, 16 +/- 3 in MeCN, and very unusual isotopic activation parameters, E(aD) - E(aH) = -2.6 +/- 0.4 and log(A(H)/A(D)) = 3.1 +/- 0.6. Computational studies, using POLYRATE, also indicate substantial tunneling in the (CH(3))(2)NO(*) + (CH(3))(2)NOH model reaction for the experimental self-exchange processes. Additional calculations on TEMPO((*)/H), (t)Bu(2)NO((*)/H), and Ph(2)NO((*)/H) self-exchange reactions reveal why the phenyl groups make the last of these reactions several orders of magnitude faster than the first two. By inference, the calculations also suggest why tunneling appears to be more important in the self-exchange reactions of dialkylhydroxylamines than of arylhydroxylamines.
Figures



Similar articles
-
Hydrogen atom transfer reactions of a ruthenium imidazole complex: hydrogen tunneling and the applicability of the Marcus cross relation.J Am Chem Soc. 2008 Nov 5;130(44):14745-54. doi: 10.1021/ja805067h. Epub 2008 Oct 9. J Am Chem Soc. 2008. PMID: 18841973 Free PMC article.
-
Slow hydrogen atom transfer reactions of oxo- and hydroxo-vanadium compounds: the importance of intrinsic barriers.J Am Chem Soc. 2009 Apr 8;131(13):4729-43. doi: 10.1021/ja808698x. J Am Chem Soc. 2009. PMID: 19292442 Free PMC article.
-
Understanding hydrogen atom transfer: from bond strengths to Marcus theory.Acc Chem Res. 2011 Jan 18;44(1):36-46. doi: 10.1021/ar100093z. Epub 2010 Oct 26. Acc Chem Res. 2011. PMID: 20977224 Free PMC article.
-
The use of cyclic nitroxide radicals as HNO scavengers.J Inorg Biochem. 2013 Jan;118:155-61. doi: 10.1016/j.jinorgbio.2012.10.002. Epub 2012 Oct 12. J Inorg Biochem. 2013. PMID: 23122928
-
Understanding Biological Hydrogen Transfer Through the Lens of Temperature Dependent Kinetic Isotope Effects.Acc Chem Res. 2018 Sep 18;51(9):1966-1974. doi: 10.1021/acs.accounts.8b00226. Epub 2018 Aug 28. Acc Chem Res. 2018. PMID: 30152685 Free PMC article. Review.
Cited by
-
Competition H(D) kinetic isotope effects in the autoxidation of hydrocarbons.J Am Chem Soc. 2015 Jan 14;137(1):94-7. doi: 10.1021/ja511434j. Epub 2014 Dec 31. J Am Chem Soc. 2015. PMID: 25533605 Free PMC article.
-
Separating Proton and Electron Transfer Effects in Three-Component Concerted Proton-Coupled Electron Transfer Reactions.J Am Chem Soc. 2017 Aug 2;139(30):10312-10319. doi: 10.1021/jacs.7b03562. Epub 2017 Jul 21. J Am Chem Soc. 2017. PMID: 28671470 Free PMC article.
-
A Simple Marcus-Theory Type Model for Hydrogen Atom Transfer/Proton-Coupled Electron Transfer.J Phys Chem Lett. 2011;2:1481-1489. doi: 10.1021/jz200021y. J Phys Chem Lett. 2011. PMID: 21686056 Free PMC article.
-
Proton-coupled electron transfer.Chem Rev. 2007 Nov;107(11):5004-64. doi: 10.1021/cr0500030. Chem Rev. 2007. PMID: 17999556 Free PMC article. Review. No abstract available.
-
Kinetic Isotope Effects and Hydrogen Tunnelling in PCET Oxidations of Ascorbate: New Insights into Aqueous Chemistry?Molecules. 2020 Mar 23;25(6):1443. doi: 10.3390/molecules25061443. Molecules. 2020. PMID: 32210039 Free PMC article. Review.
References
-
- Hynes JT, Klinman JP, Limbach H-H, Schowen RL, editors. Hydrogen-Transfer Reactions. Wiley-VCH; Weinheim: 2007.
-
-
Hammes-Schiffer S. pp. 479–502.Fernandez-Ramos A, Ellingson BA, Garrett BC, Truhlar DG. In: Reviews in Computational Chemistry. Cundari TR, Lipkowitz KB, editors. Vol. 23. Wiley-VCH; Hoboken, NJ: 2007. pp. 125–232. In ref.
-
-
- Bell RP. The Tunnel Effect in Chemistry. Chapman and Hall; London: 1980. pp. 77–105.
-
- Carpenter BK. Determination of Organic Reaction Mechanisms. John Wiley & Sons; 1984. pp. 83–111.
-
- cf.
- Klinman JP. Phil. Trans. R. Soc. B. 2006;361:1323. - PMC - PubMed
- Sutcliffe MJ, Masgrau L, Roujeinikova A, Johannissen LO, Hothi P, Basran J, Ranaghan KE, Mulholland AJ, Leys D, Scrutton NS. Phil. Trans. R. Soc. B. 2006;361:1375. - PMC - PubMed
- Barroso M, Arnaut LG, Formosinho SJ. J. Phys. Org. Chem. 2009;22:254–263.
- Meyer TJ, Huynh MHV. Inorg. Chem. 2003;42:8140–8160. - PubMed
- Reinaudt OM, Theopold KH. J. Am. Chem. Soc. 1994;116:6979–6980.
- Mahapatra S, Halfen JA, Tolman WB. J. Am. Chem. Soc. 1996;118:11575–11586.
- Zheng H, Lipscomb JD. Biochemistry. 2006;45:1685–1692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

