Sox17 regulates organ lineage segregation of ventral foregut progenitor cells
- PMID: 19619492
- PMCID: PMC2734336
- DOI: 10.1016/j.devcel.2009.05.012
Sox17 regulates organ lineage segregation of ventral foregut progenitor cells
Abstract
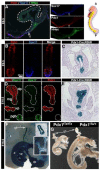
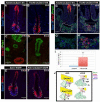
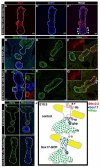
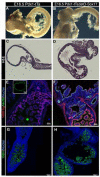
The ventral pancreas, biliary system, and liver arise from the posterior ventral foregut, but the cell-intrinsic pathway by which these organ lineages are separated is not known. Here we show that the extrahepatobiliary system shares a common origin with the ventral pancreas and not the liver, as previously thought. These pancreatobiliary progenitor cells coexpress the transcription factors PDX1 and SOX17 at E8.5 and their segregation into a PDX1+ ventral pancreas and a SOX17+ biliary primordium is Sox17-dependent. Deletion of Sox17 at E8.5 results in the loss of biliary structures and ectopic pancreatic tissue in the liver bud and common duct, while Sox17 overexpression suppresses pancreas development and promotes ectopic biliary-like tissue throughout the PDX1+ domain. Restricting SOX17+ biliary progenitor cells to the ventral region of the gut requires the notch effector Hes1. Our results highlight the role of Sox17 and Hes1 in patterning and morphogenetic segregation of ventral foregut lineages.
Figures



Similar articles
-
Ectopic pancreas formation in Hes1 -knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas.J Clin Invest. 2006 Jun;116(6):1484-93. doi: 10.1172/JCI27704. Epub 2006 May 18. J Clin Invest. 2006. PMID: 16710472 Free PMC article.
-
Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary.Nature. 2019 Oct;574(7776):112-116. doi: 10.1038/s41586-019-1598-0. Epub 2019 Sep 25. Nature. 2019. PMID: 31554966 Free PMC article.
-
Conversion of biliary system to pancreatic tissue in Hes1-deficient mice.Nat Genet. 2004 Jan;36(1):83-7. doi: 10.1038/ng1273. Epub 2003 Dec 14. Nat Genet. 2004. PMID: 14702043
-
Genetic lineage tracing, a powerful tool to investigate the embryonic organogenesis and adult organ maintenance of the pancreas.J Hepatobiliary Pancreat Sci. 2011 Jan;18(1):1-5. doi: 10.1007/s00534-010-0307-z. J Hepatobiliary Pancreat Sci. 2011. PMID: 20668890 Review.
-
Multipotent stem cells in the biliary tree.Ital J Anat Embryol. 2010;115(1-2):85-90. Ital J Anat Embryol. 2010. PMID: 21072995 Review.
Cited by
-
Development of extrahepatic bile ducts and mechanisms of tumorigenesis: Lessons from mouse models.Pathol Int. 2022 Dec;72(12):589-605. doi: 10.1111/pin.13287. Epub 2022 Nov 9. Pathol Int. 2022. PMID: 36349994 Free PMC article. Review.
-
High-Dimensional Design-Of-Experiments Extracts Small-Molecule-Only Induction Conditions for Dorsal Pancreatic Endoderm from Pluripotency.iScience. 2020 Aug 21;23(8):101346. doi: 10.1016/j.isci.2020.101346. Epub 2020 Jul 4. iScience. 2020. PMID: 32745983 Free PMC article.
-
A Dynamic WNT/β-CATENIN Signaling Environment Leads to WNT-Independent and WNT-Dependent Proliferation of Embryonic Intestinal Progenitor Cells.Stem Cell Reports. 2016 Nov 8;7(5):826-839. doi: 10.1016/j.stemcr.2016.09.004. Epub 2016 Oct 6. Stem Cell Reports. 2016. PMID: 27720905 Free PMC article.
-
SOX17 regulates uterine epithelial-stromal cross-talk acting via a distal enhancer upstream of Ihh.Nat Commun. 2018 Oct 24;9(1):4421. doi: 10.1038/s41467-018-06652-w. Nat Commun. 2018. PMID: 30356064 Free PMC article.
-
The septum transversum mesenchyme induces gall bladder development.Biol Open. 2013 Jun 20;2(8):779-88. doi: 10.1242/bio.20135348. eCollection 2013 Aug 15. Biol Open. 2013. PMID: 23951403 Free PMC article.
References
-
- Ashraf A, Abdullatif H, Hardin W, Moates JM. Unusual case of neonatal diabetes mellitus due to congenital pancreas agenesis. Pediatric Diabetes. 2005;6:239–243. - PubMed
-
- Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–5117. - PubMed
-
- Bort R, Martinez-Barbera JP, Beddington RS, Zaret KS. Hex homeobox gene-dependent tissue positioning is required for organogenesis of the ventral pancreas. Development. 2004;131:797–806. - PubMed
-
- Bort R, Signore M, Tremblay K, Martinez Barbera JP, Zaret KS. Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Developmental Biology. 2006;290:44–56. - PubMed
-
- Braunstein EM, Qiao XT, Madison B, Pinson K, Dunbar L, Gumucio DL. Villin: A marker for development of the epithelial pyloric border. Dev Dyn. 2002;224:90–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

