The large-conductance Ca(2+)-activated K(+) channel interacts with the apolipoprotein ApoA1
- PMID: 19619511
- PMCID: PMC2757790
- DOI: 10.1016/j.bbrc.2009.07.074
The large-conductance Ca(2+)-activated K(+) channel interacts with the apolipoprotein ApoA1
Abstract
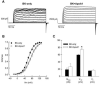
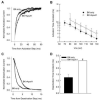
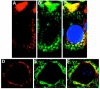
Owing to the multifaceted functions of the large conductance Ca(2+)-activated K(+) channel (BK), identification of protein-protein interactions is essential in determining BK regulation. A yeast two-hybrid screening of a cochlear cDNA library revealed a BK-ApoA1 interaction. Patch clamp recordings of excised membrane patches from transfected HEK293 cells showed that ApoA1 inhibits the BK alpha-subunit by significantly increasing activation and deactivation times, and shifting half-activation voltage to more positive potentials. Reciprocal coimmunoprecipitations verified the BK-ApoA1 interaction using excised sensory epithelium and ganglia. Additionally, immunocolocalization studies revealed BK and ApoA1 expression in both receptor cells and auditory neurons. These data suggest new avenues of investigation, given the importance of apolipoproteins in neurological diseases.
Figures
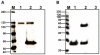
Similar articles
-
Tamoxifen inhibits BK channels in chick cochlea without alterations in voltage-dependent activation.Am J Physiol Cell Physiol. 2009 Jul;297(1):C75-85. doi: 10.1152/ajpcell.00659.2008. Epub 2009 May 13. Am J Physiol Cell Physiol. 2009. PMID: 19439526 Free PMC article.
-
CDK5 interacts with Slo and affects its surface expression and kinetics through direct phosphorylation.Am J Physiol Cell Physiol. 2012 Mar 1;302(5):C766-80. doi: 10.1152/ajpcell.00339.2011. Epub 2011 Nov 16. Am J Physiol Cell Physiol. 2012. PMID: 22094329 Free PMC article.
-
Expression of BK-type calcium-activated potassium channel splice variants during chick cochlear development.J Comp Neurol. 2010 Jul 1;518(13):2554-69. doi: 10.1002/cne.22352. J Comp Neurol. 2010. PMID: 20503427 Free PMC article.
-
Hair cell BK channels interact with RACK1, and PKC increases its expression on the cell surface by indirect phosphorylation.Am J Physiol Cell Physiol. 2012 Jul 15;303(2):C143-50. doi: 10.1152/ajpcell.00062.2012. Epub 2012 Apr 25. Am J Physiol Cell Physiol. 2012. PMID: 22538239 Free PMC article.
-
The LRRC family of BK channel regulatory subunits: potential roles in health and disease.J Physiol. 2022 Mar;600(6):1357-1371. doi: 10.1113/JP281952. Epub 2022 Jan 24. J Physiol. 2022. PMID: 35014034 Free PMC article. Review.
Cited by
-
Conserved BK channel-protein interactions reveal signals relevant to cell death and survival.PLoS One. 2011;6(12):e28532. doi: 10.1371/journal.pone.0028532. Epub 2011 Dec 9. PLoS One. 2011. PMID: 22174833 Free PMC article.
-
Bile Acid Application in Cell-Targeting for Molecular Receptors in Relation to Hearing: A Comprehensive Review.Curr Drug Targets. 2024;25(3):158-170. doi: 10.2174/0113894501278292231223035733. Curr Drug Targets. 2024. PMID: 38192136 Review.
-
Developmental expression of BK channels in chick cochlear hair cells.BMC Dev Biol. 2009 Dec 15;9:67. doi: 10.1186/1471-213X-9-67. BMC Dev Biol. 2009. PMID: 20003519 Free PMC article.
References
-
- Maruyama Y, Shimada H, Taniguchi J. Ca(2+)-activated K(+)-channels in the nuclear envelope isolated from single pancreatic acinar cells. Pflügers Arch. 1995;430:148–150. - PubMed
-
- Yamashita M, Sugioka M, Ogawa Y. Voltage- and Ca2+-activated potassium channels in Ca2+ store control Ca2+ release. FEBS J. 2006;273:3585–3597. - PubMed
-
- Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991;541:163–166. - PubMed
-
- Guo Y, Zhang C, Du X, Nair U, Yoo TJ. Morphological and functional alterations of the cochlea in apolipoprotein E gene deficient mice. Hear Res. 2005;208:54–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

