Molecular determinants of cardiac transient outward potassium current (I(to)) expression and regulation
- PMID: 19619557
- PMCID: PMC2813406
- DOI: 10.1016/j.yjmcc.2009.07.013
Molecular determinants of cardiac transient outward potassium current (I(to)) expression and regulation
Abstract
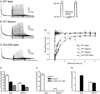
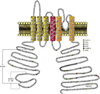
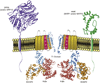
Rapidly activating and inactivating cardiac transient outward K(+) currents, I(to), are expressed in most mammalian cardiomyocytes, and contribute importantly to the early phase of action potential repolarization and to plateau potentials. The rapidly recovering (I(t)(o,f)) and slowly recovering (I(t)(o,s)) components are differentially expressed in the myocardium, contributing to regional heterogeneities in action potential waveforms. Consistent with the marked differences in biophysical properties, distinct pore-forming (alpha) subunits underlie the two I(t)(o) components: Kv4.3/Kv4.2 subunits encode I(t)(o,f), whereas Kv1.4 encodes I(t)(o,s), channels. It has also become increasingly clear that cardiac I(t)(o) channels function as components of macromolecular protein complexes, comprising (four) Kvalpha subunits and a variety of accessory subunits and regulatory proteins that influence channel expression, biophysical properties and interactions with the actin cytoskeleton, and contribute to the generation of normal cardiac rhythms. Derangements in the expression or the regulation of I(t)(o) channels in inherited or acquired cardiac diseases would be expected to increase the risk of potentially life-threatening cardiac arrhythmias. Indeed, a recently identified Brugada syndrome mutation in KCNE3 (MiRP2) has been suggested to result in increased I(t)(o,f) densities. Continued focus in this area seems certain to provide new and fundamentally important insights into the molecular determinants of functional I(t)(o) channels and into the molecular mechanisms involved in the dynamic regulation of I(t)(o) channel functioning in the normal and diseased myocardium.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Accessory Kvbeta1 subunits differentially modulate the functional expression of voltage-gated K+ channels in mouse ventricular myocytes.Circ Res. 2005 Mar 4;96(4):451-8. doi: 10.1161/01.RES.0000156890.25876.63. Epub 2005 Jan 20. Circ Res. 2005. PMID: 15662035
-
Regulation of human cardiac potassium channels by full-length KCNE3 and KCNE4.Sci Rep. 2016 Dec 6;6:38412. doi: 10.1038/srep38412. Sci Rep. 2016. PMID: 27922120 Free PMC article.
-
Molecular basis of transient outward K+ current diversity in mouse ventricular myocytes.J Physiol. 1999 Dec 15;521 Pt 3(Pt 3):587-99. doi: 10.1111/j.1469-7793.1999.00587.x. J Physiol. 1999. PMID: 10601491 Free PMC article.
-
Pharmacological Approaches for the Modulation of the Potassium Channel KV4.x and KChIPs.Int J Mol Sci. 2021 Jan 31;22(3):1419. doi: 10.3390/ijms22031419. Int J Mol Sci. 2021. PMID: 33572566 Free PMC article. Review.
-
Heterogeneous expression of voltage-gated potassium channels in the heart: roles in normal excitation and arrhythmias.J Cardiovasc Electrophysiol. 2002 Apr;13(4):406-9. doi: 10.1046/j.1540-8167.2002.00406.x. J Cardiovasc Electrophysiol. 2002. PMID: 12033361 Review.
Cited by
-
Transmural gradients in ion channel and auxiliary subunit expression.Prog Biophys Mol Biol. 2016 Dec;122(3):165-186. doi: 10.1016/j.pbiomolbio.2016.09.012. Epub 2016 Oct 1. Prog Biophys Mol Biol. 2016. PMID: 27702655 Free PMC article. Review.
-
Inhibition of BKCa channels protects neonatal hearts against myocardial ischemia and reperfusion injury.Cell Death Discov. 2022 Apr 7;8(1):175. doi: 10.1038/s41420-022-00980-z. Cell Death Discov. 2022. PMID: 35393410 Free PMC article.
-
Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes as Models for Cardiac Channelopathies: A Primer for Non-Electrophysiologists.Circ Res. 2018 Jul 6;123(2):224-243. doi: 10.1161/CIRCRESAHA.118.311209. Circ Res. 2018. PMID: 29976690 Free PMC article. Review.
-
Epicardial origin of cardiac arrhythmias: clinical evidences and pathophysiology.Cardiovasc Res. 2022 Jun 22;118(7):1693-1702. doi: 10.1093/cvr/cvab213. Cardiovasc Res. 2022. PMID: 34152392 Free PMC article. Review.
-
Effects of allitridum on the transient outward potassium current in rats with heart failure.J Geriatr Cardiol. 2016 Sep;13(9):783-788. doi: 10.11909/j.issn.1671-5411.2016.09.005. J Geriatr Cardiol. 2016. PMID: 27899943 Free PMC article.
References
-
- Dudel J, Peper K, Rudel R, Trautwein W. The dynamic chloride component of membrane current in Purkinje fibers. Pflügers Arch Gesamte Physiol Menschen Tiere. 1967;295:197–212. - PubMed
-
- Coraboeuf E, Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflügers Arch. 1982;392:352–359. - PubMed
-
- Siegelbaum SA, Tsien RW, Kass RS. Role of intracellular calcium in the transient outward current of calf Purkinje fibres. Nature. 1977;269:611–613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

