T cell epitope: friend or foe? Immunogenicity of biologics in context
- PMID: 19619593
- PMCID: PMC7103283
- DOI: 10.1016/j.addr.2009.07.001
T cell epitope: friend or foe? Immunogenicity of biologics in context
Abstract
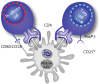
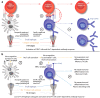
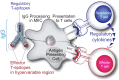
Like vaccines, biologic proteins can be very immunogenic for reasons including route of administration, dose frequency and the underlying antigenicity of the therapeutic protein. Because the impact of immunogenicity can be quite severe, regulatory agencies are developing risk-based guidelines for immunogenicity screening. T cell epitopes are at the root of the immunogenicity issue. Through their presentation to T cells, they activate the process of anti-drug antibody development. Preclinical screening for T cell epitopes can be performed in silico, followed by in vitro and in vivo validation. Importantly, screening for immunogenicity is complicated by the discovery of regulatory T cell epitopes, which suggests that immunogenicity testing must now take regulatory T cells into consideration. In this review, we address the application of computational tools for preclinical immunogenicity assessment, the implication of the discovery of regulatory T cell epitopes, and experimental validation of those assessments.
Figures



Similar articles
-
In silico methods for immunogenicity risk assessment and human homology screening for therapeutic antibodies.MAbs. 2024 Jan-Dec;16(1):2333729. doi: 10.1080/19420862.2024.2333729. Epub 2024 Mar 27. MAbs. 2024. PMID: 38536724 Free PMC article.
-
T-cell dependent immunogenicity of protein therapeutics: Preclinical assessment and mitigation.Clin Immunol. 2013 Dec;149(3):534-55. doi: 10.1016/j.clim.2013.09.006. Epub 2013 Sep 25. Clin Immunol. 2013. PMID: 24263283 Review.
-
Specificity of the T Cell Response to Protein Biopharmaceuticals.Front Immunol. 2020 Jul 22;11:1550. doi: 10.3389/fimmu.2020.01550. eCollection 2020. Front Immunol. 2020. PMID: 32793213 Free PMC article. Review.
-
Prediction of immunogenicity for therapeutic proteins: state of the art.Curr Opin Drug Discov Devel. 2007 May;10(3):332-40. Curr Opin Drug Discov Devel. 2007. PMID: 17554860 Review.
-
Fundamentals and Methods for T- and B-Cell Epitope Prediction.J Immunol Res. 2017;2017:2680160. doi: 10.1155/2017/2680160. Epub 2017 Dec 28. J Immunol Res. 2017. PMID: 29445754 Free PMC article. Review.
Cited by
-
SILVI, an open-source pipeline for T-cell epitope selection.PLoS One. 2022 Sep 7;17(9):e0273494. doi: 10.1371/journal.pone.0273494. eCollection 2022. PLoS One. 2022. PMID: 36070252 Free PMC article.
-
Low immunogenicity predicted for emerging avian-origin H7N9: implication for influenza vaccine design.Hum Vaccin Immunother. 2013 May;9(5):950-6. doi: 10.4161/hv.24939. Epub 2013 May 1. Hum Vaccin Immunother. 2013. PMID: 23807079 Free PMC article.
-
Better Epitope Discovery, Precision Immune Engineering, and Accelerated Vaccine Design Using Immunoinformatics Tools.Front Immunol. 2020 Apr 7;11:442. doi: 10.3389/fimmu.2020.00442. eCollection 2020. Front Immunol. 2020. PMID: 32318055 Free PMC article. Review.
-
Quantitative analysis of the CD4+ T cell response to therapeutic antibodies in healthy donors using a novel T cell:PBMC assay.PLoS One. 2017 May 31;12(5):e0178544. doi: 10.1371/journal.pone.0178544. eCollection 2017. PLoS One. 2017. PMID: 28562666 Free PMC article.
-
iVAX: An integrated toolkit for the selection and optimization of antigens and the design of epitope-driven vaccines.Hum Vaccin Immunother. 2015;11(9):2312-21. doi: 10.1080/21645515.2015.1061159. Hum Vaccin Immunother. 2015. PMID: 26155959 Free PMC article.
References
-
- De Groot A.S., Scott D.W. Immunogenicity of protein therapeutics. Trends. Immunol. 2007;28:482–490. - PubMed
-
- Zubler R.H. Naive and memory B cells in T-cell-dependent and T-independent responses. Springer. Semin. Immunopathol. 2001;23:405–419. - PubMed
-
- Koren E., Zuckerman L.A., Mire-Sluis A.R. Immune responses to therapeutic proteins in humans— clinical significance, assessment and prediction. Curr. Pharm. Biotechnol. 2002;3:349–360. - PubMed
-
- Casadevall N., Nataf J., Viron B., Kolta A., Kiladjian J.J., Martin-Dupont P., Michaud P., Papo T., Ugo V., Teyssandier I., Varet B., Mayeux P. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N. Engl. J. Med. 2002;346:469–475. - PubMed
-
- Koren E., Smith H.W., Shores E., Shankar G., Finco-Kent D., Rup B., Barrett Y.C., Devanarayan V., Gorovits B., Gupta S., Parish T., Quarmby V., Moxness M., Swanson S.J., Taniguchi G., Zuckerman L.A., Stebbins C.C., Mire-Sluis A. Recommendations on risk-based strategies for detection and characterization of antibodies against biotechnology products. J. Immunol. Methods. 2008;333:1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

