Depurinating naphthalene-DNA adducts in mouse skin related to cancer initiation
- PMID: 19619639
- PMCID: PMC4424927
- DOI: 10.1016/j.freeradbiomed.2009.07.020
Depurinating naphthalene-DNA adducts in mouse skin related to cancer initiation
Abstract
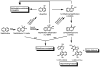
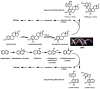
Naphthalene has been shown to be a weak carcinogen in rats. To investigate its mechanism of metabolic activation and cancer initiation, mice were topically treated with naphthalene or one of its metabolites, 1-naphthol, 1,2-dihydrodiolnaphthalene (1,2-DDN), 1,2-dihydroxynaphthalene (1,2-DHN), and 1,2-naphthoquinone (1,2-NQ). After 4 h, the mice were sacrificed, the treated skin was excised, and the depurinating and stable DNA adducts were analyzed. The depurinating adducts were identified and quantified by ultraperformance liquid chromatography/tandem mass spectrometry, whereas the stable adducts were quantified by (32)P-postlabeling. For comparison, the stable adducts formed when a mixture of the four deoxyribonucleoside monophosphates was treated with 1,2-NQ or enzyme-activated naphthalene were also analyzed. The depurinating adducts 1,2-DHN-1-N3Ade and 1,2-DHN-1-N7Gua arise from reaction of 1,2-NQ with DNA. Similarly, the major stable adducts appear to derive from the 1,2-NQ. The depurinating DNA adducts are, in general, the most abundant. Therefore, naphthalene undergoes metabolic activation to the electrophilic ortho-quinone, 1,2-NQ, which reacts with DNA to form depurinating adducts. This is the same mechanism as other weak carcinogens, such as the natural and synthetic estrogens, and benzene.
Figures
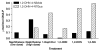

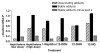
Similar articles
-
Formation of depurinating N3adenine and N7guanine adducts after reaction of 1,2-naphthoquinone or enzyme-activated 1,2-dihydroxynaphthalene with DNA. Implications for the mechanism of tumor initiation by naphthalene.Chem Biol Interact. 2007 Feb 20;165(3):175-88. doi: 10.1016/j.cbi.2006.12.007. Epub 2006 Dec 16. Chem Biol Interact. 2007. PMID: 17224140
-
Expanded analysis of benzo[a]pyrene-DNA adducts formed in vitro and in mouse skin: their significance in tumor initiation.Chem Res Toxicol. 1996 Jul-Aug;9(5):897-903. doi: 10.1021/tx960004a. Chem Res Toxicol. 1996. PMID: 8828927
-
A unifying mechanism in the initiation of cancer and other diseases by catechol quinones.Ann N Y Acad Sci. 2004 Dec;1028:247-57. doi: 10.1196/annals.1322.029. Ann N Y Acad Sci. 2004. PMID: 15650250 Review.
-
Catechol quinones of estrogens in the initiation of breast, prostate, and other human cancers: keynote lecture.Ann N Y Acad Sci. 2006 Nov;1089:286-301. doi: 10.1196/annals.1386.042. Ann N Y Acad Sci. 2006. PMID: 17261777
-
The molecular etiology and prevention of estrogen-initiated cancers: Ockham's Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity.Mol Aspects Med. 2014 Apr;36:1-55. doi: 10.1016/j.mam.2013.08.002. Epub 2013 Aug 30. Mol Aspects Med. 2014. PMID: 23994691 Free PMC article. Review.
Cited by
-
Structural and kinetic characterization of recombinant 2-hydroxymuconate semialdehyde dehydrogenase from Pseudomonas putida G7.Arch Biochem Biophys. 2015 Aug 1;579:8-17. doi: 10.1016/j.abb.2015.05.006. Epub 2015 May 29. Arch Biochem Biophys. 2015. PMID: 26032336 Free PMC article.
-
Urinary naphthol metabolites and chromosomal aberrations in 5-year-old children.Cancer Epidemiol Biomarkers Prev. 2012 Jul;21(7):1191-202. doi: 10.1158/1055-9965.EPI-12-0214. Epub 2012 May 9. Cancer Epidemiol Biomarkers Prev. 2012. PMID: 22573794 Free PMC article.
-
The Photodynamic Properties and the Genotoxicity of Heat-Treated Silicalite-1 Films.Materials (Basel). 2019 Feb 14;12(4):567. doi: 10.3390/ma12040567. Materials (Basel). 2019. PMID: 30769806 Free PMC article.
-
The etiology and prevention of breast cancer.Drug Discov Today Dis Mech. 2012 Summer;9(1-2):e55-e69. doi: 10.1016/j.ddmec.2013.02.001. Drug Discov Today Dis Mech. 2012. PMID: 26246832 Free PMC article.
-
DNA adducts form in mouse lung and liver after oral naphthalene exposure.Toxicol Sci. 2025 May 1;205(1):42-46. doi: 10.1093/toxsci/kfaf017. Toxicol Sci. 2025. PMID: 39921883 Free PMC article.
References
-
- Stanley JS. Environmental Protection Agency, office of toxic substances. Vol. 1. Washington, DC: 1986. Broad scan analysis of the FY82 national human adipose tissue survey specimens, executive summary (EPA-560/5-86-035)
-
- Pellizzari ED, Hartwell TD, Harris BSH, III, Waddell RD, Whitaker DA, Erickson MD. Purgeable organic compounds in mother’s milk. Bull Environ Contam Toxicol. 1982;28:322–328. - PubMed
-
- Environmental Protection Agency. Clean air act amendments of 1990 conference report to accompany S. 1630. Office of Pollution Prevention and Toxics; Washington, DC: 1990. (Report No. 101–952)
-
- Buckpitt A, Boland B, Isbell M, Morin D, Shultz M, Baldwin R, Chan K, Karlsson A, Lin C, Taff A, West J, Fanucchi M, Van Winkle L, Plopper C. Naphthalene-induced respiratory tract toxicity: metabolic mechanisms of toxicity. Drug Metab Rev. 2002;34:791–820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

