Chronic hypoxia suppresses the CO2 response of solitary complex (SC) neurons from rats
- PMID: 19619674
- PMCID: PMC2750817
- DOI: 10.1016/j.resp.2009.07.012
Chronic hypoxia suppresses the CO2 response of solitary complex (SC) neurons from rats
Abstract
We studied the effect of chronic hypobaric hypoxia (CHx; 10-11% O(2)) on the response to hypercapnia (15% CO(2)) of individual solitary complex (SC) neurons from adult rats. We simultaneously measured the intracellular pH and firing rate responses to hypercapnia of SC neurons in superfused medullary slices from control and CHx-adapted adult rats using the blind whole cell patch clamp technique and fluorescence imaging microscopy. We found that CHx caused the percentage of SC neurons inhibited by hypercapnia to significantly increase from about 10% up to about 30%, but did not significantly alter the percentage of SC neurons activated by hypercapnia (50% in control vs. 35% in CHx). Further, the magnitudes of the responses of SC neurons from control rats (chemosensitivity index for activated neurons of 166+/-11% and for inhibited neurons of 45+/-15%) were the same in SC neurons from CHx-adapted rats. This plasticity induced in chemosensitive SC neurons by CHx appears to involve intrinsic changes in neuronal properties since they were the same in synaptic blockade medium.
Figures

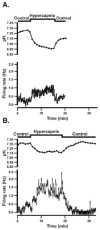

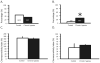


Similar articles
-
Substance P differentially modulates firing rate of solitary complex (SC) neurons from control and chronic hypoxia-adapted adult rats.PLoS One. 2014 Feb 7;9(2):e88161. doi: 10.1371/journal.pone.0088161. eCollection 2014. PLoS One. 2014. PMID: 24516602 Free PMC article.
-
Characterization of the chemosensitive response of individual solitary complex neurons from adult rats.Am J Physiol Regul Integr Comp Physiol. 2009 Mar;296(3):R763-73. doi: 10.1152/ajpregu.90769.2008. Epub 2009 Jan 14. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19144749 Free PMC article.
-
Development of chemosensitivity in neurons from the nucleus tractus solitarii (NTS) of neonatal rats.Respir Physiol Neurobiol. 2009 Mar 31;166(1):4-12. doi: 10.1016/j.resp.2008.11.005. Epub 2008 Nov 14. Respir Physiol Neurobiol. 2009. PMID: 19056522 Free PMC article.
-
[Chemosensitivity of the neuronal membrane (identifiable giant neurons of the Aplysia)].Mars Med. 1969;106(11):843-9. Mars Med. 1969. PMID: 4947061 Review. French. No abstract available.
-
Intracellular pH regulation of neurons in chemosensitive and nonchemosensitive areas of brain slices.Respir Physiol. 2001 Dec;129(1-2):37-56. doi: 10.1016/s0034-5687(01)00281-x. Respir Physiol. 2001. PMID: 11738645 Review.
Cited by
-
Time Domains of the Hypoxic Ventilatory Response and Their Molecular Basis.Compr Physiol. 2016 Jun 13;6(3):1345-85. doi: 10.1002/cphy.c150026. Compr Physiol. 2016. PMID: 27347896 Free PMC article. Review.
-
Ibuprofen blocks time-dependent increases in hypoxic ventilation in rats.Respir Physiol Neurobiol. 2011 Sep 30;178(3):381-6. doi: 10.1016/j.resp.2011.03.024. Epub 2011 Mar 30. Respir Physiol Neurobiol. 2011. PMID: 21457799 Free PMC article.
-
Nucleus Tractus Solitarius Neurons Activated by Hypercapnia and Hypoxia Lack Mu Opioid Receptor Expression.Front Mol Neurosci. 2022 Jul 11;15:932189. doi: 10.3389/fnmol.2022.932189. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35898697 Free PMC article.
-
Sustained Hypoxia Alters nTS Glutamatergic Signaling and Expression and Function of Excitatory Amino Acid Transporters.Neuroscience. 2020 Mar 15;430:131-140. doi: 10.1016/j.neuroscience.2020.01.034. Epub 2020 Feb 4. Neuroscience. 2020. PMID: 32032667 Free PMC article.
-
Ventilatory effects of substance P-saporin lesions in the nucleus tractus solitarii of chronically hypoxic rats.Am J Physiol Regul Integr Comp Physiol. 2011 Aug;301(2):R343-50. doi: 10.1152/ajpregu.00375.2010. Epub 2011 May 18. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21593425 Free PMC article.
References
-
- Barnard P, Andronikou S, Pokorski M, Smatresk N, Mokashi A, Lahiri S. Time-dependent effect of hypoxia on carotid body chemosensory function. J Appl Physiol. 1987;63:685–691. - PubMed
-
- Bisgard GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York, Basel, Hong Kong: Marcel Dekker, Inc.; 1995. pp. 617–618.
-
- Bisgard GE. Carotid body mechanisms in acclimatization to hypoxia. Respir Physiol. 2000;121:237–46. - PubMed
-
- Chung S, Ivy GO, Reid SG. GABA-mediated neurotransmission in the nucleus of the solitary tract alters resting ventilation following exposure to chronic hypoxia in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2006:R1449–R1456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

