When to start antiretroviral therapy in resource-limited settings
- PMID: 19620143
- PMCID: PMC3092478
- DOI: 10.7326/0003-4819-151-3-200908040-00138
When to start antiretroviral therapy in resource-limited settings
Abstract
Background: The results of international clinical trials that are assessing when to initiate antiretroviral therapy (ART) will not be available for several years.
Objective: To inform HIV treatment decisions about the optimal CD4 threshold at which to initiate ART in South Africa while awaiting the results of these trials.
Design: Cost-effectiveness analysis by using a computer simulation model of HIV disease.
Data sources: Published data from randomized trials and observational cohorts in South Africa.
Target population: HIV-infected patients in South Africa.
Time horizon: 5-year and lifetime.
Perspective: Modified societal.
Intervention: No treatment, ART initiated at a CD4 count less than 0.250 x 10(9) cells/L, and ART initiated at a CD4 count less than 0.350 x 10(9) cells/L.
Outcome measures: Morbidity, mortality, life expectancy, medical costs, and cost-effectiveness.
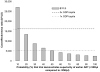
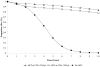
Results of base-case analysis: If 10% to 100% of HIV-infected patients are identified and linked to care, a CD4 count threshold for ART initiation of 0.350 x 10(9) cells/L would reduce severe opportunistic diseases by 22,000 to 221,000 and deaths by 25,000 to 253,000 during the next 5 years compared with ART initiation at 0.250 x 10(9) cells/L; cost increases would range from $142 million (10%) to $1.4 billion (100%). Either ART initiation strategy would increase long-term survival by at least 7.9 years, with a mean per-person life expectancy of 3.8 years with no ART and 12.5 years with an initiation threshold of 0.350 x 10(9) cells/L. Compared with an initiation threshold of 0.250 x 10(9) cells/L, a threshold of 0.350 x 10(9) cells/L has an incremental cost-effectiveness ratio of $1200 per year of life saved.
Results of sensitivity analysis: Initiating ART at a CD4 count less than 0.350 x 10(9) cells/L would remain cost-effective over the next 5 years even if the probability that the trial would demonstrate the superiority of earlier therapy is as low as 17%.
Limitation: This model does not consider the possible benefits of initiating ART at a CD4 count greater than 0.350 x 10(9) cells/L or of reduced HIV transmission.
Conclusion: Earlier initiation of ART in South Africa will probably reduce morbidity and mortality, improve long-term survival, and be cost-effective. While awaiting trial results, treatment guidelines should be liberalized to allow initiation at CD4 counts less than 0.350 x 10(9) cells/L, earlier than is currently recommended.
Primary funding source: National Institute of Allergy and Infectious Diseases and the Doris Duke Charitable Foundation.
Figures

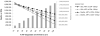
Comment in
-
Modeling comparative effectiveness and the value of research.Ann Intern Med. 2009 Aug 4;151(3):210-1. doi: 10.7326/0003-4819-151-3-200908040-00010. Ann Intern Med. 2009. PMID: 19652188 No abstract available.
References
-
- Sterne Jonathan, et al. When should HIV-1-infected persons initiate ART? Collaborative analysis of HIV cohort studies [abstract 72LB]. 16th Conference on Retroviruses and Opportunistic Infections; Montréal, QC, CA. 2009.
-
- Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197(8):1133–44. - PubMed
-
- Kitahata M, Gange S, Moore R, et al. Initiating rather than deferring HAART at a CD4+ count >500 cells/mm3 is associated with improved survival [abstract 71]. 16th Conference on Retroviruses and Opportunistic Infections; Montréal, QC, Canada. 2009.
-
- Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42(4):464–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
