Intrinsic voltage dependence of the epithelial Na+ channel is masked by a conserved transmembrane domain tryptophan
- PMID: 19620245
- PMCID: PMC2757952
- DOI: 10.1074/jbc.M109.015917
Intrinsic voltage dependence of the epithelial Na+ channel is masked by a conserved transmembrane domain tryptophan
Abstract
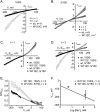
Tryptophan residues critical to function are frequently located at the lipid-water interface of transmembrane domains. All members of the epithelial Na+ channel (ENaC)/Degenerin (Deg) channel superfamily contain an absolutely conserved Trp at the base of their first transmembrane domain. Here, we test the importance of this conserved Trp to ENaC/Deg function. Targeted substitution of this Trp in mouse ENaC and rat ASIC subunits decrease channel activity. Differential substitution with distinct amino acids in alpha-mENaC shows that it is loss of this critical Trp rather than introduction of residues having novel properties that changes channel activity. Surprisingly, Trp substitution unmasks voltage sensitivity. Mutant ENaC has increased steady-state activity at hyperpolarizing compared with depolarizing potentials associated with transient activation and deactivation times, respectively. The times of activation and deactivation change 1 ms/mV in a linear manner with rising and decreasing slopes, respectively. Increases in macroscopic currents at hyperpolarizing potentials results from a voltage-dependent increase in open probability. Voltage sensitivity is not influenced by divalent cations; however, it is Na+-dependent with a 63-mV decrease in voltage required to reach half-maximal activity per log increase in [Na+]. Mutant channels are particularly sensitive to intracellular [Na+] for removing this sodium abolishes voltage dependence. We conclude that the conserved Trp at the base of TM1 in ENaC/Deg channels protects against voltage by masking an inhibitory allosteric or pore block mechanism, which decreases activity in response to intracellular Na+.
Figures






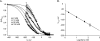

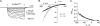
References
-
- Kellenberger S., Schild L. (2002) Physiol. Rev. 82, 735–767 - PubMed
-
- Garty H., Palmer L. G. (1997) Physiol. Rev. 77, 359–396 - PubMed
-
- Bianchi L., Driscoll M. (2002) Neuron 34, 337–340 - PubMed
-
- Lifton R. P., Gharavi A. G., Geller D. S. (2001) Cell 104, 545–556 - PubMed
-
- Bonny O., Hummler E. (2000) Kidney Int. 57, 1313–1318 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

