Novel molecular determinants in the pore region of sodium channels regulate local anesthetic binding
- PMID: 19620257
- PMCID: PMC2769053
- DOI: 10.1124/mol.109.055863
Novel molecular determinants in the pore region of sodium channels regulate local anesthetic binding
Abstract
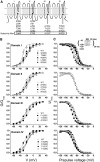
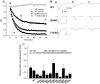
The pore of the Na+ channel is lined by asymmetric loops formed by the linkers between the fifth and sixth transmembrane segments (S5-S6). We investigated the role of the N-terminal portion (SS1) of the S5-S6 linkers in channel gating and local anesthetic (LA) block using site-directed cysteine mutagenesis of the rat skeletal muscle (Na(V)1.4) channel. The mutants examined have variable effects on voltage dependence and kinetics of fast inactivation. Of the cysteine mutants immediately N-terminal to the putative DEKA selectivity filter in four domains, only Q399C in domain I and F1236C in domain III exhibit reduced use-dependent block. These two mutations also markedly accelerated the recovery from use-dependent block. Moreover, F1236C and Q399C significantly decreased the affinity of QX-314 for binding to its channel receptor by 8.5- and 3.3-fold, respectively. Oddly enough, F1236C enhanced stabilization of slow inactivation by both hastening entry into and delaying recovery from slow inactivation states. It is noteworthy that symmetric applications of QX-314 on both external and internal sides of F1236C mutant channels reduced recovery from use-dependent block, indicating an allosteric effect of external QX-314 binding on the recovery of availability of F1236C. These observations suggest that cysteine mutation in the SS1 region, particularly immediate adjacent to the DEKA ring, may lead to a structural rearrangement that alters binding of permanently charged QX-314 to its receptor. The results lend further support for a role for the selectivity filter region as a structural determinant for local anesthetic block.
Figures
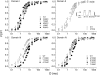




Similar articles
-
Altered gating and local anesthetic block mediated by residues in the I-S6 and II-S6 transmembrane segments of voltage-dependent Na+ channels.Mol Pharmacol. 2003 Sep;64(3):741-52. doi: 10.1124/mol.64.3.741. Mol Pharmacol. 2003. PMID: 12920212
-
Outward stabilization of the S4 segments in domains III and IV enhances lidocaine block of sodium channels.J Physiol. 2007 Jul 1;582(Pt 1):317-34. doi: 10.1113/jphysiol.2007.134262. Epub 2007 May 17. J Physiol. 2007. PMID: 17510181 Free PMC article.
-
Quaternary ammonium block of mutant Na+ channels lacking inactivation: features of a transition-intermediate mechanism.J Physiol. 2000 Nov 15;529 Pt 1(Pt 1):93-106. doi: 10.1111/j.1469-7793.2000.00093.x. J Physiol. 2000. PMID: 11080254 Free PMC article.
-
Interactions of local anesthetics with voltage-gated Na+ channels.J Membr Biol. 2004 Sep 1;201(1):1-8. doi: 10.1007/s00232-004-0702-y. J Membr Biol. 2004. PMID: 15635807 Review.
-
Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review.
Cited by
-
A molecular switch between the outer and the inner vestibules of the voltage-gated Na+ channel.J Biol Chem. 2010 Dec 10;285(50):39458-70. doi: 10.1074/jbc.M110.132886. Epub 2010 Oct 6. J Biol Chem. 2010. PMID: 20926383 Free PMC article.
-
Mechanism of sodium channel block by local anesthetics, antiarrhythmics, and anticonvulsants.J Gen Physiol. 2017 Apr 3;149(4):465-481. doi: 10.1085/jgp.201611668. Epub 2017 Mar 3. J Gen Physiol. 2017. PMID: 28258204 Free PMC article.
-
The outer vestibule of the Na+ channel-toxin receptor and modulator of permeation as well as gating.Mar Drugs. 2010 Apr 21;8(4):1373-93. doi: 10.3390/md8041373. Mar Drugs. 2010. PMID: 20479982 Free PMC article. Review.
-
Opposing Effects on NaV1.2 Function Underlie Differences Between SCN2A Variants Observed in Individuals With Autism Spectrum Disorder or Infantile Seizures.Biol Psychiatry. 2017 Aug 1;82(3):224-232. doi: 10.1016/j.biopsych.2017.01.009. Epub 2017 Jan 27. Biol Psychiatry. 2017. PMID: 28256214 Free PMC article.
-
Local anesthetic and antiepileptic drug access and binding to a bacterial voltage-gated sodium channel.Proc Natl Acad Sci U S A. 2014 Sep 9;111(36):13057-62. doi: 10.1073/pnas.1408710111. Epub 2014 Aug 18. Proc Natl Acad Sci U S A. 2014. PMID: 25136136 Free PMC article.
References
-
- Ahern CA, Eastwood AL, Dougherty DA, Horn R. (2008) Electrostatic contributions of aromatic residues in the local anesthetic receptor of voltage-gated sodium channels. Circ Res 102:86–94 - PubMed
-
- Alpert LA, Fozzard HA, Hanck DA, Makielski JC. (1989) Is there a second external lidocaine binding site on mammalian cardiac cells? Am J Physiol 257:H79–H84 - PubMed
-
- Baumgarten CM, Makielski JC, Fozzard HA. (1991) External site for local anesthetic block of cardiac Na+ channels. J Mol Cell Cardiol 23 (Suppl 1):85–93 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

