Vaccine-induced antibody isotypes are skewed by impaired CD4 T cell and invariant NKT cell effector responses in MyD88-deficient mice
- PMID: 19620295
- PMCID: PMC4454352
- DOI: 10.4049/jimmunol.0804011
Vaccine-induced antibody isotypes are skewed by impaired CD4 T cell and invariant NKT cell effector responses in MyD88-deficient mice
Abstract
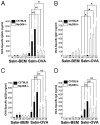
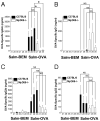
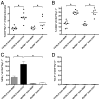
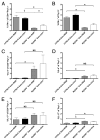
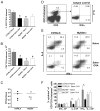
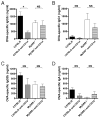
The requirement for TLR signaling in the initiation of an Ag-specific Ab response is controversial. In this report we show that a novel OVA-expressing recombinant Salmonella vaccine (Salmonella-OVA) elicits a Th1-biased cell-mediated and serum Ab response upon oral or i.p. immunization of C57BL/6 mice. In MyD88(-/-) mice, Th1-dependent Ab responses are greatly reduced while Th2-dependent Ab isotypes are elevated in response to oral and i.p., but not s.c. footpad, immunization. When the T effector response to oral vaccination is examined we find that activated, adoptively transferred Ag-specific CD4(+) T cells accumulate in the draining lymph nodes, but fail to produce IFN-gamma, in MyD88(-/-) mice. Moreover, CD1d tetramer staining shows that invariant NKT cells are activated in response to oral Salmonella-OVA vaccination in wild-type, but not MyD88(-/-), mice. Treatment with neutralizing Ab to CD1d reduces the OVA-specific Ab response only in MyD88-sufficient wild-type mice, suggesting that both Ag-specific CD4 T cell and invariant NKT cell effector responses to Salmonella-OVA vaccination are MyD88 dependent. Taken together, our data indicate that the type of adaptive immune response generated to this live attenuated vaccine is regulated by both the presence of MyD88-mediated signals and vaccination route, which may have important implications for future vaccine design.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783– 801. - PubMed
-
- Krieg AM. Toll-free vaccines? Nat Biotechnol. 2007;25:303–305. - PubMed
-
- Lanzavecchia A, Sallusto F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol. 2007;19:268–274. - PubMed
-
- Pulendran B. Tolls and beyond: many roads to vaccine immunity. N Engl J Med. 2007;356:1776–1778. - PubMed
-
- Wickelgren I. Immunology: mouse studies question importance of Toll-like receptors to vaccines. Science. 2006;314:1859–1860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

