Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis
- PMID: 19620331
- PMCID: PMC2764177
- DOI: 10.1128/AAC.00830-09
Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis
Abstract
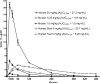
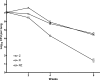
To investigate the antagonism between isoniazid (INH) and rifampin (rifampicin) (RIF)-pyrazinamide (PZA) combination observed in Mycobacterium tuberculosis-infected mice, extensive pharmacokinetic studies of INH were performed and followed by experiments to assess the impact of increasing doses of INH on the antimicrobial activity of RIF-PZA combination. INH at 6.25 mg/kg of body weight produced a maximum concentration of drug in serum (Cmax) value of 4 microg/ml and an area under the concentration-time curve from 0 to 24 h (AUC(0-24)) value of 4.9 microg x h/ml, the former being close to the Cmax value observed after the standard 5-mg/kg dose in humans. INH at 25 mg/kg produced a Cmax value of 22 microg/ml and an AUC(0-24) value of 29 microg x h/ml, the latter being close to the AUC observed after a 5-mg/kg dose of INH in humans with the slow acetylation phenotype. Beginning 2 weeks after aerosol infection with M. tuberculosis, mice were treated for 8 weeks with INH at twofold-increasing doses, ranging from 1.56 to 50 mg/kg, either alone or in combination with RIF-PZA. Given alone, INH exhibited dose-dependent activity. Combined with RIF-PZA, INH exhibited dose-dependent antagonism of RIF-PZA activity. To determine the individual components of RIF-PZA combination with which INH was antagonistic, mice were treated for 8 weeks with RIF alone, PZA alone, RIF-PZA, and INH at 3.125, 12.5, or 50 mg/kg either alone or combined with RIF or PZA. Addition of INH to RIF had additive activity, whereas addition of INH to PZA resulted in a negative interaction. Finally, a 10-mg/kg dose of INH in mice may best represent the 5-mg/kg dose in humans and decrease the antagonism of INH with RIF-PZA.
Figures

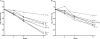
References
-
- Blumberg, H. M., W. J. Burman, R. E. Chaisson, C. L. Daley, S. C. Etkind, L. N. Friedman, P. Fujiwara, M. Grzemska, P. C. Hopewell, M. D. Iseman, R. M. Jasmer, V. Koppaka, R. I. Menzies, R. J. O'Brien, R. R. Reves, L. B. Reichman, P. M. Simone, J. R. Starke, and A. A. Vernon for the American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America. 2003. Treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167:603-662. - PubMed
-
- Dorman, S. E., J. L. Johnson, S. Goldberg, G. Muzanye, N. Padayatchi, L. Bozeman, C. M. Heilig, J. Bernardo, S. Choudhri, J. H. Grosset, E. Guy, P. Guyadeen, M. C. Leus, G. Maltas, D. Menzies, E. L. Nuermberger, M. Villarino, A. Vernon, and R. E. Chaisson. 30 April 2009. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. doi:10.1164/rccm.200901-0078OC. - DOI - PubMed
-
- East African/British Medical Research Council. 1972. Controlled clinical trial of short-course (6-month) regimens of chemotherapy for treatment of pulmonary tuberculosis. Lancet i:1079-1085. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

