Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model
- PMID: 19620341
- PMCID: PMC2747916
- DOI: 10.1128/IAI.00592-09
Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model
Abstract
We previously described that low-dose Mycobacterium tuberculosis infection in cynomolgus macaques results in a spectrum of disease similar to that of human infection: primary disease, latent infection, and reactivation tuberculosis (S. V. Capuano III, D. A. Croix, S. Pawar, A. Zinovik, A. Myers, P. L. Lin, S. Bissel, C. Fuhrman, E. Klein, and J. L. Flynn, Infect. Immun. 71:5831-5844, 2003). This is the only established model of latent infection, and it provides a unique opportunity to understand host and pathogen differences across of range of disease states. Here, we provide a more extensive and detailed characterization of the gross pathology, microscopic histopathology, and immunologic characteristics of monkeys in each clinical disease category. The data underscore the similarities between human and nonhuman primate M. tuberculosis infection. Furthermore, we describe novel methods of quantifying gross pathology and bacterial burden that distinguish between active disease and latent infection, and we extend the usefulness of this model for comparative studies. Early in infection, an abnormal chest X ray, M. tuberculosis growth by gastric aspirate, and increased mycobacterium-specific gamma interferon (IFN-gamma) in peripheral blood mononuclear cells (PBMCs) and bronchoalveolar lavage (BAL) cells were associated with the development of active disease. At necropsy, disease was quantified with respect to pathology and bacterial numbers. Microscopically, a spectrum of granuloma types are seen and differ with disease type. At necropsy, monkeys with active disease had more lung T cells and more IFN-gamma from PBMC, BAL, and mediastinal lymph nodes than monkeys with latent infection. Finally, we have observed a spectrum of disease not only in monkeys with active disease but also in those with latent infection that provides insight into human latent tuberculosis.
Figures
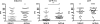


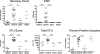



References
-
- Barnes, P. F., T. D. Verdegem, L. A. Vachon, J. M. Leedom, and G. D. Overturf. 1988. Chest roentgenogram in pulmonary tuberculosis. New data on an old test. Chest 94:316-320. - PubMed
-
- Canetti, G. 1955. The tubercle bacillus. Springer Publishing Co., Inc., New York, NY.
-
- Capuano, S. V., III, D. A. Croix, S. Pawar, A. Zinovik, A. Myers, P. L. Lin, S. Bissel, C. Fuhrman, E. Klein, and J. L. Flynn. 2003. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect. Immun. 71:5831-5844. - PMC - PubMed
-
- Chee, C. B., T. M. Barkham, K. W. Khinmar, S. H. Gan, and Y. T. Wang. 2008. Quantitative T-cell interferon-gamma responses to Mycobacterium tuberculosis-specific antigens in active and latent tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 28:667-670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

