Role of Nod1 in mucosal dendritic cells during Salmonella pathogenicity island 1-independent Salmonella enterica serovar Typhimurium infection
- PMID: 19620349
- PMCID: PMC2747964
- DOI: 10.1128/IAI.00519-09
Role of Nod1 in mucosal dendritic cells during Salmonella pathogenicity island 1-independent Salmonella enterica serovar Typhimurium infection
Erratum in
- Infect Immun. 2009 Nov;77(11):5203
Abstract
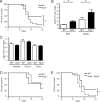
Recent advances in immunology have highlighted the critical function of pattern-recognition molecules (PRMs) in generating the innate immune response to effectively target pathogens. Nod1 and Nod2 are intracellular PRMs that detect peptidoglycan motifs from the cell walls of bacteria once they gain access to the cytosol. Salmonella enterica serovar Typhimurium is an enteric intracellular pathogen that causes a severe disease in the mouse model. This pathogen resides within vacuoles inside the cell, but the question of whether cytosolic PRMs such as Nod1 and Nod2 could have an impact on the course of S. Typhimurium infection in vivo has not been addressed. Here, we show that deficiency in the PRM Nod1, but not Nod2, resulted in increased susceptibility toward a mutant strain of S. Typhimurium that targets directly lamina propria dendritic cells (DCs) for its entry into the host. Using this bacterium and bone marrow chimeras, we uncovered a surprising role for Nod1 in myeloid cells controlling bacterial infection at the level of the intestinal lamina propria. Indeed, DCs deficient for Nod1 exhibited impaired clearance of the bacteria, both in vitro and in vivo, leading to increased organ colonization and decreased host survival after oral infection. Taken together, these findings demonstrate a key role for Nod1 in the host response to an enteric bacterial pathogen through the modulation of intestinal lamina propria DCs.
Figures

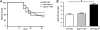

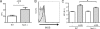
References
-
- Bajaj, V., R. L. Lucas, C. Hwang, and C. A. Lee. 1996. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol. Microbiol. 22:703-714. - PubMed
-
- Brennan, M. A., and B. T. Cookson. 2000. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38:31-40. - PubMed
-
- Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:702-707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

