A truncated CFTR protein rescues endogenous DeltaF508-CFTR and corrects chloride transport in mice
- PMID: 19620404
- PMCID: PMC2775001
- DOI: 10.1096/fj.08-127878
A truncated CFTR protein rescues endogenous DeltaF508-CFTR and corrects chloride transport in mice
Abstract
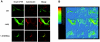
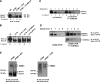
Cystic fibrosis (CF) is most frequently associated with deletion of phenylalanine at position 508 (DeltaF508) in the CF transmembrane conductance regulator (CFTR) protein. The DeltaF508-CFTR mutant protein exhibits a folding defect that affects its processing and impairs chloride-channel function. This study aimed to determine whether CFTR fragments approximately half the size of wild-type CFTR and complementary to the portion of CFTR bearing the mutation can specifically rescue the processing of endogenous DeltaF508-CFTR in vivo. cDNA encoding CFTR fragments were delivered to human airway epithelial cells and mice harboring endogenous DeltaF508-CFTR. Delivery of small CFTR fragments, which do not act as chloride channels by themselves, rescue DeltaF508-CFTR. Therefore, we can speculate that the presence of the CFTR fragment, which does not harbor a mutation, might facilitate intermolecular interactions. The rescue of CFTR was evident by the restoration of chloride transport in human CFBE41o- bronchial epithelial cells expressing DeltaF508-CFTR in vitro. More important, nasal administration of an adenovirus expressing a complementary CFTR fragment restored some degree of CFTR activity in the nasal airways of DeltaF508 homozygous mice in vivo. These findings identify complementary protein fragments as a viable in vivo approach for correcting disease-causing misfolding of plasma membrane proteins.
Figures
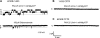



Similar articles
-
Tgf-β1 inhibits Cftr biogenesis and prevents functional rescue of ΔF508-Cftr in primary differentiated human bronchial epithelial cells.PLoS One. 2013 May 9;8(5):e63167. doi: 10.1371/journal.pone.0063167. Print 2013. PLoS One. 2013. PMID: 23671668 Free PMC article.
-
Activation of the cystic fibrosis transmembrane conductance regulator by the flavonoid quercetin: potential use as a biomarker of ΔF508 cystic fibrosis transmembrane conductance regulator rescue.Am J Respir Cell Mol Biol. 2010 Nov;43(5):607-16. doi: 10.1165/rcmb.2009-0281OC. Epub 2009 Dec 30. Am J Respir Cell Mol Biol. 2010. PMID: 20042712 Free PMC article.
-
Low temperature and chemical rescue affect molecular proximity of DeltaF508-cystic fibrosis transmembrane conductance regulator (CFTR) and epithelial sodium channel (ENaC).J Biol Chem. 2012 May 11;287(20):16781-90. doi: 10.1074/jbc.M111.332031. Epub 2012 Mar 22. J Biol Chem. 2012. PMID: 22442149 Free PMC article.
-
CFTR: folding, misfolding and correcting the ΔF508 conformational defect.Trends Mol Med. 2012 Feb;18(2):81-91. doi: 10.1016/j.molmed.2011.10.003. Epub 2011 Dec 3. Trends Mol Med. 2012. PMID: 22138491 Free PMC article. Review.
-
Can correcting the ΔF508-CFTR proteostasis-defect rescue CF lung disease?Curr Mol Med. 2012 Aug;12(7):860-71. doi: 10.2174/156652412801318773. Curr Mol Med. 2012. PMID: 22697346 Review.
Cited by
-
Targeted Integration of a Super-Exon into the CFTR Locus Leads to Functional Correction of a Cystic Fibrosis Cell Line Model.PLoS One. 2016 Aug 15;11(8):e0161072. doi: 10.1371/journal.pone.0161072. eCollection 2016. PLoS One. 2016. PMID: 27526025 Free PMC article.
-
Dysferlin-peptides reallocate mutated dysferlin thereby restoring function.PLoS One. 2012;7(11):e49603. doi: 10.1371/journal.pone.0049603. Epub 2012 Nov 20. PLoS One. 2012. PMID: 23185377 Free PMC article.
-
Functional rescue of a misfolded eukaryotic ATP-binding cassette transporter by domain replacement.J Biol Chem. 2010 Nov 12;285(46):36225-34. doi: 10.1074/jbc.M110.160523. Epub 2010 Sep 14. J Biol Chem. 2010. PMID: 20843810 Free PMC article.
-
Transcomplementation by a truncation mutant of cystic fibrosis transmembrane conductance regulator (CFTR) enhances ΔF508 processing through a biomolecular interaction.J Biol Chem. 2013 Apr 12;288(15):10505-12. doi: 10.1074/jbc.M112.420489. Epub 2013 Mar 5. J Biol Chem. 2013. PMID: 23463513 Free PMC article.
-
Probing conformational rescue induced by a chemical corrector of F508del-cystic fibrosis transmembrane conductance regulator (CFTR) mutant.J Biol Chem. 2011 Jul 15;286(28):24714-25. doi: 10.1074/jbc.M111.239699. Epub 2011 May 21. J Biol Chem. 2011. PMID: 21602569 Free PMC article.
References
-
- Kopito R R. Biosynthesis and degradation of CFTR. Physiol Rev. 1999;79:S167–173. - PubMed
-
- Tsui L C. The spectrum of cystic fibrosis mutations. Trends Genet. 1992;8:392–398. - PubMed
-
- Jensen T J, Loo M A, Pind S, Williams D B, Goldberg A L, Riordan J R. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell. 1995;83:129–135. - PubMed
-
- Gelman M S, Kannegaard E S, Kopito R R. A principal role for the proteasome in endoplasmic reticulum-associated degradation of misfolded intracellular cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:11709–11714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

