An outer arm dynein light chain acts in a conformational switch for flagellar motility
- PMID: 19620633
- PMCID: PMC2717645
- DOI: 10.1083/jcb.200905083
An outer arm dynein light chain acts in a conformational switch for flagellar motility
Abstract
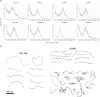
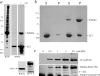
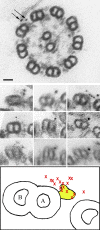
A system distinct from the central pair-radial spoke complex was proposed to control outer arm dynein function in response to alterations in the mechanical state of the flagellum. In this study, we examine the role of a Chlamydomonas reinhardtii outer arm dynein light chain that associates with the motor domain of the gamma heavy chain (HC). We demonstrate that expression of mutant forms of LC1 yield dominant-negative effects on swimming velocity, as the flagella continually beat out of phase and stall near or at the power/recovery stroke switchpoint. Furthermore, we observed that LC1 interacts directly with tubulin in a nucleotide-independent manner and tethers this motor unit to the A-tubule of the outer doublet microtubules within the axoneme. Therefore, this dynein HC is attached to the same microtubule by two sites: via both the N-terminal region and the motor domain. We propose that this gamma HC-LC1-microtubule ternary complex functions as a conformational switch to control outer arm activity.
Figures







Similar articles
-
The complex of outer-arm dynein light chain-1 and the microtubule-binding domain of the γ heavy chain shows how axonemal dynein tunes ciliary beating.J Biol Chem. 2020 Mar 20;295(12):3982-3989. doi: 10.1074/jbc.RA119.011541. Epub 2020 Feb 3. J Biol Chem. 2020. PMID: 32014992 Free PMC article.
-
Stuck in reverse: loss of LC1 in Trypanosoma brucei disrupts outer dynein arms and leads to reverse flagellar beat and backward movement.J Cell Sci. 2007 May 1;120(Pt 9):1513-20. doi: 10.1242/jcs.004846. Epub 2007 Apr 3. J Cell Sci. 2007. PMID: 17405810
-
The LC7 light chains of Chlamydomonas flagellar dyneins interact with components required for both motor assembly and regulation.Mol Biol Cell. 2004 Oct;15(10):4633-46. doi: 10.1091/mbc.e04-06-0461. Epub 2004 Aug 10. Mol Biol Cell. 2004. PMID: 15304520 Free PMC article.
-
Sensing the mechanical state of the axoneme and integration of Ca2+ signaling by outer arm dynein.Cytoskeleton (Hoboken). 2010 Apr;67(4):207-13. doi: 10.1002/cm.20445. Cytoskeleton (Hoboken). 2010. PMID: 20186692 Free PMC article. Review.
-
Functional diversity of axonemal dyneins as studied in Chlamydomonas mutants.Int Rev Cytol. 2002;219:115-55. doi: 10.1016/s0074-7696(02)19012-7. Int Rev Cytol. 2002. PMID: 12211628 Review.
Cited by
-
Outer-arm dynein light chain LC1 is required for normal motor assembly kinetics, ciliary stability, and motility.Mol Biol Cell. 2023 Jun 1;34(7):ar75. doi: 10.1091/mbc.E23-03-0104. Epub 2023 May 3. Mol Biol Cell. 2023. PMID: 37133971 Free PMC article.
-
Functional architecture of the outer arm dynein conformational switch.J Biol Chem. 2012 Jan 27;287(5):3108-22. doi: 10.1074/jbc.M111.286211. Epub 2011 Dec 7. J Biol Chem. 2012. PMID: 22157010 Free PMC article.
-
A solid-state control system for dynein-based ciliary/flagellar motility.J Cell Biol. 2013 Apr 15;201(2):173-5. doi: 10.1083/jcb.201302077. Epub 2013 Apr 8. J Cell Biol. 2013. PMID: 23569213 Free PMC article.
-
Schmidtea mediterranea as a Model Organism to Study the Molecular Background of Human Motile Ciliopathies.Int J Mol Sci. 2023 Feb 24;24(5):4472. doi: 10.3390/ijms24054472. Int J Mol Sci. 2023. PMID: 36901899 Free PMC article. Review.
-
The complex of outer-arm dynein light chain-1 and the microtubule-binding domain of the γ heavy chain shows how axonemal dynein tunes ciliary beating.J Biol Chem. 2020 Mar 20;295(12):3982-3989. doi: 10.1074/jbc.RA119.011541. Epub 2020 Feb 3. J Biol Chem. 2020. PMID: 32014992 Free PMC article.
References
-
- Baron D.M., Kabututu Z.P., Hill K.L. 2007. Stuck in reverse: loss of LC1 in Trypanosoma brucei disrupts outer dynein arms and leads to reverse flagellar beat and backward movement.J. Cell Sci. 120:1513–1520 - PubMed
-
- Bell C.W., Fronk E., Gibbons I.R. 1979. Polypeptide subunits of dynein 1 from sea urchin sperm flagella.J. Supramol. Struct. 11:311–317 - PubMed
-
- Benashski S.E., Patel-King R.S., King S.M. 1999. Light chain 1 from the Chlamydomonas outer dynein arm is a leucine-rich repeat protein associated with the motor domain of the γ heavy chain.Biochemistry.38:7253–7264 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

