Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells
- PMID: 19620635
- PMCID: PMC2717640
- DOI: 10.1083/jcb.200901021
Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells
Abstract
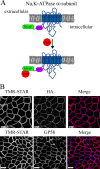
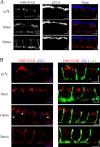
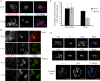
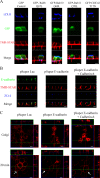
Newly synthesized apical and basolateral membrane proteins are sorted from one another in polarized epithelial cells. The trans-Golgi network participates in this sorting process, but some basolateral proteins travel from the Golgi to recycling endosomes (REs) before their surface delivery. Using a novel system for pulse-chase microscopy, we have visualized the postsynthetic route pursued by a newly synthesized cohort of Na,K-ATPase. We find that the basolateral delivery of newly synthesized Na,K-ATPase occurs via a pathway distinct from that pursued by the vesicular stomatitis virus G protein (VSV-G). Na,K-ATPase surface delivery occurs at a faster rate than that observed for VSV-G. The Na,K-ATPase does not pass through the RE compartment en route to the plasma membrane, and Na,K-ATPase trafficking is not regulated by the same small GTPases as other basolateral proteins. Finally, Na,K-ATPase and VSV-G travel in separate post-Golgi transport intermediates, demonstrating directly that multiple routes exist for transport from the Golgi to the basolateral membrane in polarized epithelial cells.
Figures


Similar articles
-
Dual pulse-chase microscopy reveals early divergence in the biosynthetic trafficking of the Na,K-ATPase and E-cadherin.Mol Biol Cell. 2015 Dec 1;26(24):4401-11. doi: 10.1091/mbc.E14-09-1385. Epub 2015 Sep 30. Mol Biol Cell. 2015. PMID: 26424804 Free PMC article.
-
Vectorial insertion of apical and basolateral membrane proteins in polarized epithelial cells revealed by quantitative 3D live cell imaging.J Cell Biol. 2006 Mar 27;172(7):1035-44. doi: 10.1083/jcb.200512012. J Cell Biol. 2006. PMID: 16567501 Free PMC article.
-
Ankyrin facilitates intracellular trafficking of alpha1-Na+-K+-ATPase in polarized cells.Am J Physiol Cell Physiol. 2008 Nov;295(5):C1202-14. doi: 10.1152/ajpcell.00273.2008. Epub 2008 Sep 3. Am J Physiol Cell Physiol. 2008. PMID: 18768923 Free PMC article.
-
Trafficking to the apical and basolateral membranes in polarized epithelial cells.J Am Soc Nephrol. 2014 Jul;25(7):1375-86. doi: 10.1681/ASN.2013080883. Epub 2014 Mar 20. J Am Soc Nephrol. 2014. PMID: 24652803 Free PMC article. Review.
-
Taking the scenic route: biosynthetic traffic to the plasma membrane in polarized epithelial cells.Traffic. 2009 Aug;10(8):972-81. doi: 10.1111/j.1600-0854.2009.00927.x. Epub 2009 May 12. Traffic. 2009. PMID: 19453969 Free PMC article. Review.
Cited by
-
A 3-D cell culture system to study epithelia functions using microcarriers.Cytotechnology. 2016 Oct;68(5):1813-25. doi: 10.1007/s10616-015-9935-0. Epub 2016 Feb 4. Cytotechnology. 2016. PMID: 26847791 Free PMC article.
-
Kidney epithelial cells are active mechano-biological fluid pumps.Nat Commun. 2022 Apr 28;13(1):2317. doi: 10.1038/s41467-022-29988-w. Nat Commun. 2022. PMID: 35484146 Free PMC article.
-
Basolateral delivery of the type I transforming growth factor beta receptor is mediated by a dominant-acting cytoplasmic motif.Mol Biol Cell. 2017 Oct 1;28(20):2701-2711. doi: 10.1091/mbc.E17-05-0334. Epub 2017 Aug 2. Mol Biol Cell. 2017. PMID: 28768825 Free PMC article.
-
Rab5 is necessary for the biogenesis of the endolysosomal system in vivo.Nature. 2012 May 23;485(7399):465-70. doi: 10.1038/nature11133. Nature. 2012. PMID: 22622570
-
Identification of Cellular Genes Involved in Baculovirus GP64 Trafficking to the Plasma Membrane.J Virol. 2022 Jun 22;96(12):e0021522. doi: 10.1128/jvi.00215-22. Epub 2022 May 24. J Virol. 2022. PMID: 35608346 Free PMC article.
References
-
- Bok D., O'Day W., Rodriguez-Boulan E. 1992. Polarized budding of vesicular stomatitis and influenza virus from cultured human and bovine retinal pigment epithelium.Exp. Eye Res. 55:853–860 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

