Chlorine activation indoors and outdoors via surface-mediated reactions of nitrogen oxides with hydrogen chloride
- PMID: 19620710
- PMCID: PMC2713392
- DOI: 10.1073/pnas.0904195106
Chlorine activation indoors and outdoors via surface-mediated reactions of nitrogen oxides with hydrogen chloride
Erratum in
- Proc Natl Acad Sci U S A. 2009 Sep 29;106(39):16889
Abstract
Gaseous HCl generated from a variety of sources is ubiquitous in both outdoor and indoor air. Oxides of nitrogen (NO(y)) are also globally distributed, because NO formed in combustion processes is oxidized to NO(2), HNO(3), N(2)O(5) and a variety of other nitrogen oxides during transport. Deposition of HCl and NO(y) onto surfaces is commonly regarded as providing permanent removal mechanisms. However, we show here a new surface-mediated coupling of nitrogen oxide and halogen activation cycles in which uptake of gaseous NO(2) or N(2)O(5) on solid substrates generates adsorbed intermediates that react with HCl to generate gaseous nitrosyl chloride (ClNO) and nitryl chloride (ClNO(2)), respectively. These are potentially harmful gases that photolyze to form highly reactive chlorine atoms. The reactions are shown both experimentally and theoretically to be enhanced by water, a surprising result given the availability of competing hydrolysis reaction pathways. Airshed modeling incorporating HCl generated from sea salt shows that in coastal urban regions, this heterogeneous chemistry increases surface-level ozone, a criteria air pollutant, greenhouse gas and source of atmospheric oxidants. In addition, it may contribute to recently measured high levels of ClNO(2) in the polluted coastal marine boundary layer. This work also suggests the potential for chlorine atom chemistry to occur indoors where significant concentrations of oxides of nitrogen and HCl coexist.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




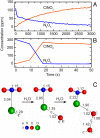

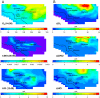
Comment in
-
Are chlorine atoms significant tropospheric free radicals?Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):13639-40. doi: 10.1073/pnas.0907089106. Epub 2009 Aug 12. Proc Natl Acad Sci U S A. 2009. PMID: 19706493 Free PMC article. No abstract available.
Similar articles
-
A large atomic chlorine source inferred from mid-continental reactive nitrogen chemistry.Nature. 2010 Mar 11;464(7286):271-4. doi: 10.1038/nature08905. Nature. 2010. PMID: 20220847
-
Surface-catalyzed chlorine and nitrogen activation: mechanisms for the heterogeneous formation of ClNO, NO, NO2, HONO, and N2O from HNO3 and HCl on aluminum oxide particle surfaces.J Phys Chem A. 2012 May 31;116(21):5180-92. doi: 10.1021/jp301488b. Epub 2012 May 17. J Phys Chem A. 2012. PMID: 22536987
-
Nitryl chloride and molecular chlorine in the coastal marine boundary layer.Environ Sci Technol. 2012 Oct 2;46(19):10463-70. doi: 10.1021/es204632r. Epub 2012 Apr 6. Environ Sci Technol. 2012. PMID: 22443276
-
Chemical reactions among indoor pollutants: what we've learned in the new millennium.Indoor Air. 2004;14 Suppl 7:184-94. doi: 10.1111/j.1600-0668.2004.00287.x. Indoor Air. 2004. PMID: 15330786 Review.
-
The atmospheric chemistry of indoor environments.Environ Sci Process Impacts. 2020 Jan 1;22(1):25-48. doi: 10.1039/c9em00386j. Epub 2019 Nov 12. Environ Sci Process Impacts. 2020. PMID: 31712796 Review.
Cited by
-
Are chlorine atoms significant tropospheric free radicals?Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):13639-40. doi: 10.1073/pnas.0907089106. Epub 2009 Aug 12. Proc Natl Acad Sci U S A. 2009. PMID: 19706493 Free PMC article. No abstract available.
-
Interactive effects of atmospheric oxidising pollutants and heat waves on the risk of residential mortality.Glob Health Action. 2024 Dec 31;17(1):2313340. doi: 10.1080/16549716.2024.2313340. Epub 2024 Feb 21. Glob Health Action. 2024. PMID: 38381455 Free PMC article.
-
Bioactive Compounds Isolated from Microalgae in Chronic Inflammation and Cancer.Mar Drugs. 2015 Sep 30;13(10):6152-209. doi: 10.3390/md13106152. Mar Drugs. 2015. PMID: 26437418 Free PMC article. Review.
-
Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards.Proc Natl Acad Sci U S A. 2010 Apr 13;107(15):6576-81. doi: 10.1073/pnas.0912820107. Epub 2010 Feb 8. Proc Natl Acad Sci U S A. 2010. PMID: 20142504 Free PMC article.
-
Oxygenated VOCs, aqueous chemistry, and potential impacts on residential indoor air composition.Indoor Air. 2018 Jan;28(1):198-212. doi: 10.1111/ina.12422. Epub 2017 Sep 20. Indoor Air. 2018. PMID: 28833580 Free PMC article.
References
-
- Keene WC. In: Naturally-Produced Organohalogens. Grimvall A, Leer EWBd, editors. Dordrecht, The Netherlands: Kluwer Academic; 1995. pp. 363–373.
-
- Loupa G, Charpantidou E, Karageorgos E, Rapsomanikis S. The chemistry of gaseous acids in medieval churches in Cyprus. Atmos Environ. 2007;41:9018–9029.
-
- Loupa G, Rapsomanikis S. Air pollutant emission rates and concentrations in medieval churches. J Atmos Chem. 2008;60:169–187.
-
- Pszenny AAP, et al. Evidence of inorganic chlorine gases other than hydrogen chloride in marine surface air. Geophys Res Lett. 1993;20:699–702.
-
- Keene WC, et al. Composite global emissions of reactive chlorine from anthropogenic and natural sources: Reactive chlorine emissions inventory. J Geophys Res. 1999;104:8429–8440.

