Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation
- PMID: 19620717
- PMCID: PMC2722274
- DOI: 10.1073/pnas.0905195106
Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation
Abstract
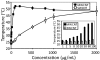
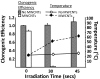
Multiwalled carbon nanotubes (MWCNTs) exhibit physical properties that render them ideal candidates for application as noninvasive mediators of photothermal cancer ablation. Here, we demonstrate that use of MWCNTs to generate heat in response to near-infrared radiation (NIR) results in thermal destruction of kidney cancer in vitro and in vivo. We document the thermal effects of the therapy through magnetic resonance temperature-mapping and heat shock protein-reactive immunohistochemistry. Our results demonstrate that use of MWCNTs enables ablation of tumors with low laser powers (3 W/cm(2)) and very short treatment times (a single 30-sec treatment) with minimal local toxicity and no evident systemic toxicity. These treatment parameters resulted in complete ablation of tumors and a >3.5-month durable remission in 80% of mice treated with 100 microg of MWCNT. Use of MWCNTs with NIR may be effective in anticancer therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Pommier Y, et al. Apoptosis defects and chemotherapy resistance: Molecular interaction maps and networks. Oncogene. 2004;23:2934–2949. - PubMed
-
- Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. - PubMed
-
- Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res. 2005;127:208–223. - PubMed
-
- Hildebrandt B, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56. - PubMed
-
- Imamura J, et al. Neoplastic seeding after radiofrequency ablation for hepatocellular carcinoma. Am J Gastroenterol. 2008;103:3057–3062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

