Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity
- PMID: 19620771
- PMCID: PMC2719935
- DOI: 10.1172/JCI37716
Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity
Abstract
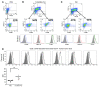
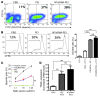
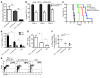
The success of clinically relevant immunotherapies requires reversing tumor-induced immunosuppression. Here we demonstrated that linear polyethylenimine-based (PEI-based) nanoparticles encapsulating siRNA were preferentially and avidly engulfed by regulatory DCs expressing CD11c and programmed cell death 1-ligand 1 (PD-L1) at ovarian cancer locations in mice. PEI-siRNA uptake transformed these DCs from immunosuppressive cells to efficient antigen-presenting cells that activated tumor-reactive lymphocytes and exerted direct tumoricidal activity, both in vivo and in situ. PEI triggered robust and selective TLR5 activation in vitro and elicited the production of hallmark TLR5-inducible cytokines in WT mice, but not in Tlr5-/- littermates. Thus, PEI is a TLR5 agonist that, to our knowledge, was not previously recognized. In addition, PEI-complexed nontargeting siRNA oligonucleotides stimulated TLR3 and TLR7. The nonspecific activation of multiple TLRs (specifically, TLR5 and TLR7) reversed the tolerogenic phenotype of human and mouse ovarian tumor-associated DCs. In ovarian carcinoma-bearing mice, this induced T cell-mediated tumor regression and prolonged survival in a manner dependent upon myeloid differentiation primary response gene 88 (MyD88; i.e., independent of TLR3). Furthermore, gene-specific siRNA-PEI nanocomplexes that silenced immunosuppressive molecules on mouse tumor-associated DCs elicited discernibly superior antitumor immunity and enhanced therapeutic effects compared with nontargeting siRNA-PEI nanocomplexes. Our results demonstrate that the intrinsic TLR5 and TLR7 stimulation of siRNA-PEI nanoparticles synergizes with the gene-specific silencing activity of siRNA to transform tumor-infiltrating regulatory DCs into DCs capable of promoting therapeutic antitumor immunity.
Figures





References
-
- Plate J.M., Harris J.E. Immune cell functions in pancreatic cancer. Crit. Rev. Immunol. 2000;20:375–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

