Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells
- PMID: 19620786
- PMCID: PMC2719942
- DOI: 10.1172/JCI37884
Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells
Abstract
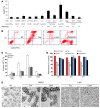
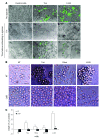
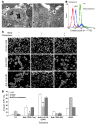
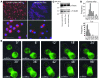
mAbs are becoming increasingly utilized in the treatment of lymphoid disorders. Although Fc-FcgammaR interactions are thought to account for much of their therapeutic effect, this does not explain why certain mAb specificities are more potent than others. An additional effector mechanism underlying the action of some mAbs is the direct induction of cell death. Previously, we demonstrated that certain CD20-specific mAbs (which we termed type II mAbs) evoke a nonapoptotic mode of cell death that appears to be linked with the induction of homotypic adhesion. Here, we reveal that peripheral relocalization of actin is critical for the adhesion and cell death induced by both the type II CD20-specific mAb tositumomab and an HLA-DR-specific mAb in both human lymphoma cell lines and primary chronic lymphocytic leukemia cells. The cell death elicited was rapid, nonapoptotic, nonautophagic, and dependent on the integrity of plasma membrane cholesterol and activation of the V-type ATPase. This cytoplasmic cell death involved lysosomes, which swelled and then dispersed their contents, including cathepsin B, into the cytoplasm and surrounding environment. The resulting loss of plasma membrane integrity occurred independently of caspases and was not controlled by Bcl-2. These experiments provide what we believe to be new insights into the mechanisms by which 2 clinically relevant mAbs elicit cell death and show that this homotypic adhesion-related cell death occurs through a lysosome-dependent pathway.
Figures





Comment in
-
Engaging the lysosomal compartment to combat B cell malignancies.J Clin Invest. 2009 Aug;119(8):2133-6. doi: 10.1172/JCI40259. Epub 2009 Jul 20. J Clin Invest. 2009. PMID: 19620776 Free PMC article.
-
Possible misinterpretation of the mode of action of therapeutic antibodies in vitro: homotypic adhesion and flow cytometry result in artefactual direct cell death.Blood. 2010 Oct 28;116(17):3372-3; author reply 3373-4. doi: 10.1182/blood-2010-06-289736. Blood. 2010. PMID: 21030571 No abstract available.
References
-
- Chan H.T., et al. CD20-induced lymphoma cell death is independent of both caspases and its redistribution into triton X-100 insoluble membrane rafts. Cancer Res. 2003;63:5480–5489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

