Modulation of mitochondrial bioenergetics in the isolated Guinea pig beating heart by potassium and lidocaine cardioplegia: implications for cardioprotection
- PMID: 19620879
- PMCID: PMC2774837
- DOI: 10.1097/FJC.0b013e3181b2b842
Modulation of mitochondrial bioenergetics in the isolated Guinea pig beating heart by potassium and lidocaine cardioplegia: implications for cardioprotection
Abstract
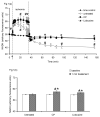
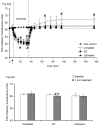
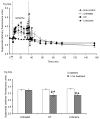
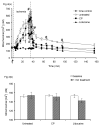
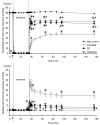
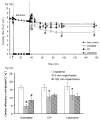
Mitochondria are damaged by cardiac ischemia/reperfusion (I/R) injury but can contribute to cardioprotection. We tested if hyperkalemic cardioplegia (CP) and lidocaine (LID) differently modulate mitochondrial (m) bioenergetics and protect hearts against I/R injury. Guinea pig hearts (n = 71) were perfused with Krebs Ringer's solution before perfusion for 1 minute just before ischemia with either CP (16 mM K) or LID (1 mM) or Krebs Ringer's (control, 4 mM K). The 1-minute perfusion period assured treatment during ischemia but not on reperfusion. Cardiac function, NADH, FAD, m[Ca], and superoxide (reactive oxygen species) were assessed at baseline, during the 1-minute perfusion, and continuously during I/R. During the brief perfusion before ischemia, CP and LID decreased reactive oxygen species and increased NADH without changing m[Ca]. Additionally, CP decreased FAD. During ischemia, NADH was higher and reactive oxygen species was lower after CP and LID, whereas m[Ca] was lower only after LID. On reperfusion, NADH and FAD were more normalized, and m[Ca] and reactive oxygen species remained lower after CP and LID. Better functional recovery and smaller infarct size after CP and LID were accompanied by better mitochondrial function. These results suggest that mitochondria may be implicated, directly or indirectly, in protection by CP and LID against I/R injury.
Conflict of interest statement
Conflict of interest: None.
Figures

References
-
- Cleveland JC, Jr, Meldrum DR, Rowland RT, Banerjee A, Harken AH. Optimal myocardial preservation: cooling, cardioplegia, and conditioning. Ann Thorac Surg. 1996;61:760–768. - PubMed
-
- Roe BB, Hutchinson JC, Fishman NH, Ullyot DJ, Smith DL. Myocardial protection with cold, ischemic, potassium-induced cardioplegia. J Thorac Cardiovasc Surg. 1977;73:366–374. - PubMed
-
- Attwell D, Cohen I, Eisner D, Ohba M, Ojeda C. The steady state TTX-sensitive (“window”) sodium current in cardiac Purkinje fibres. Pflugers Arch. 1979;379:137–142. - PubMed
-
- Jovanovic A, Alekseev AE, Lopez JR, Shen WK, Terzic A. Adenosine prevents hyperkalemia-induced calcium loading in cardiac cells: relevance for cardioplegia. Ann Thorac Surg. 1997;63:153–161. - PubMed
-
- Cohen NM, Wise RM, Wechsler AS, Damiano RJ., Jr Elective cardiac arrest with a hyperpolarizing adenosine triphosphate-sensitive potassium channel opener. A novel form of myocardial protection? J Thorac Cardiovasc Surg. 1993;106:317–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

