Evolution of efficient pathways for degradation of anthropogenic chemicals
- PMID: 19620997
- PMCID: PMC2867350
- DOI: 10.1038/nchembio.197
Evolution of efficient pathways for degradation of anthropogenic chemicals
Abstract
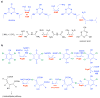
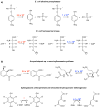
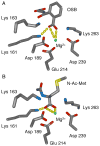
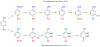
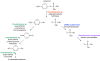
Anthropogenic compounds used as pesticides, solvents and explosives often persist in the environment and can cause toxicity to humans and wildlife. The persistence of anthropogenic compounds is due to their recent introduction into the environment; microbes in soil and water have had relatively little time to evolve efficient mechanisms for degradation of these new compounds. Some anthropogenic compounds are easily degraded, whereas others are degraded very slowly or only partially, leading to accumulation of toxic products. This review examines the factors that affect the ability of microbes to degrade anthropogenic compounds and the mechanisms by which new pathways emerge in nature. New approaches for engineering microbes with enhanced degradative abilities include assembly of pathways using enzymes from multiple organisms, directed evolution of inefficient enzymes, and genome shuffling to improve microbial fitness under the challenging conditions posed by contaminated environments.
Figures
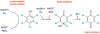
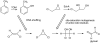
References
-
- Stockdale M, Selwyn MJ. Effects of ring substituents on the activity of phenols as inhibitors and uncouplers of mitochondrial respiration. Eur J Biochem. 1971;21:565–574. - PubMed
-
- Ting HP, Wilson DF, Chance B. Effects of uncouplers of oxidative phosphorylation on the specific conductance of bimolecular lipid membranes. Arch Biochem Biophys. 1970;141:141–6. - PubMed
-
- Badkoubi A, Stevens DK, Murarka IP. Quantification of pentachlorophenol product distribution in the presence of Phanerochaete chrysosporium. Arch Env Contam Toxicol. 1996;30:1–8.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

