CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium
- PMID: 19621064
- PMCID: PMC2705187
- DOI: 10.1371/journal.pbio.1000155
CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium
Abstract
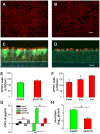
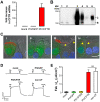
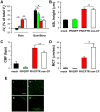
Dysfunction of CFTR in cystic fibrosis (CF) airway epithelium perturbs the normal regulation of ion transport, leading to a reduced volume of airway surface liquid (ASL), mucus dehydration, decreased mucus transport, and mucus plugging of the airways. CFTR is normally expressed in ciliated epithelial cells of the surface and submucosal gland ductal epithelium and submucosal gland acinar cells. Critical questions for the development of gene transfer strategies for CF airway disease are what airway regions require CFTR function and how many epithelial cells require CFTR expression to restore normal ASL volume regulation and mucus transport to CF airway epithelium? An in vitro model of human CF ciliated surface airway epithelium (CF HAE) was used to test whether a human parainfluenza virus (PIV) vector engineered to express CFTR (PIVCFTR) could deliver sufficient CFTR to CF HAE to restore mucus transport, thus correcting the CF phenotype. PIVCFTR delivered CFTR to >60% of airway surface epithelial cells and expressed CFTR protein in CF HAE approximately 100-fold over endogenous levels in non-CF HAE. This efficiency of CFTR delivery fully corrected the basic bioelectric defects of Cl(-) and Na(+) epithelial ion transport and restored ASL volume regulation and mucus transport to levels approaching those of non-CF HAE. To determine the numbers of CF HAE surface epithelial cells required to express CFTR for restoration of mucus transport to normal levels, different amounts of PIVCFTR were used to express CFTR in 3%-65% of the surface epithelial cells of CF HAE and correlated to increasing ASL volumes and mucus transport rates. These data demonstrate for the first time, to our knowledge, that restoration of normal mucus transport rates in CF HAE was achieved after CFTR delivery to 25% of surface epithelial cells. In vivo experimentation in appropriate models will be required to determine what level of mucus transport will afford clinical benefit to CF patients, but we predict that a future goal for corrective gene transfer to the CF human airways in vivo would attempt to target at least 25% of surface epithelial cells to achieve mucus transport rates comparable to those in non-CF airways.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Comment in
-
Engineered common cold virus helps cultured cystic fibrosis tissues clear mucus.PLoS Biol. 2009 Jul 21;7(7):e1000160. doi: 10.1371/journal.pbio.1000160. PLoS Biol. 2009. PMID: 20076750 Free PMC article. No abstract available.
References
-
- Koch C, Hoiby N. Pathogenesis of cystic fibrosis. Lancet. 1993;341:1065–1069. - PubMed
-
- Wu J. V, Krouse M. E, Wine J. J. Acinar origin of CFTR-dependent airway submucosal gland fluid secretion. Am J Physiol Lung Cell Mol Physiol. 2007;292:L304–311. - PubMed
-
- Jeffery P. Form and function of airway epithelium. In: Jones C. J, editor. Epithelia: advances in cell physiology and cell culture. London: Kluwer Academic Publishers; 1990. pp. 195–220.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

