Examination of enzymatic H-tunneling through kinetics and dynamics
- PMID: 19621965
- PMCID: PMC2746013
- DOI: 10.1021/ja902120t
Examination of enzymatic H-tunneling through kinetics and dynamics
Abstract
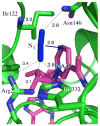
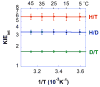
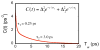
In recent years, kinetic measurements of isotope effects of enzyme-catalyzed reactions and their temperature dependence led to the development of theoretical models that were used to rationalize the findings. These models suggested that motions at the femto- to picosecond (fs to ps) time scale modulate the environment of the catalyzed reaction. Due to the fast nature of motions that directly affect the cleavage of a covalent bond, it is challenging to correlate the enzyme kinetics and dynamics related to that step. We report a study of formate dehydrogenase (FDH) that compares the temperature dependence of intrinsic kinetic isotope effects (KIEs) to measurements of the environmental dynamics at the fs-ps time scale (Bandaria et al. J. Am. Chem. Soc. 2008, 130, 22-23). The findings from this comparison of experimental kinetics and dynamics are consistent with models of environmentally coupled H-tunneling models, also known as Marcus-like models. Apparently, at tunneling ready conformations, the donor-acceptor distance, orientation, and fluctuations seems to be well tuned for H-transfer and are not affected by thermal fluctuations slower than 10 ps. This phenomenon has been suggested in the past to be quite general in enzymatic reactions. Here, the kinetics and the dynamics measurements on a single chemical step and on fs-ps time scale, respectively, provide new insight and support for the relevant theoretical models. Furthermore, this methodology could be applied to other systems and be used to examine mutants for which the organization of the donor and acceptor is not ideal, or enzymes with different rigidity and different temperature optimum.
Figures

Similar articles
-
Isotopic Labeling of Formate Dehydrogenase Perturbs the Protein Dynamics.J Phys Chem B. 2019 Dec 12;123(49):10403-10409. doi: 10.1021/acs.jpcb.9b08426. Epub 2019 Dec 2. J Phys Chem B. 2019. PMID: 31696711
-
Protein Mass Effects on Formate Dehydrogenase.J Am Chem Soc. 2017 Dec 6;139(48):17405-17413. doi: 10.1021/jacs.7b08359. Epub 2017 Nov 27. J Am Chem Soc. 2017. PMID: 29083897 Free PMC article.
-
Replication of the Enzymatic Temperature Dependency of the Primary Hydride Kinetic Isotope Effects in Solution: Caused by the Protein-Controlled Rigidity of the Donor-Acceptor Centers?Biochemistry. 2019 Oct 1;58(39):4035-4046. doi: 10.1021/acs.biochem.9b00574. Epub 2019 Sep 13. Biochemistry. 2019. PMID: 31478638
-
Understanding Biological Hydrogen Transfer Through the Lens of Temperature Dependent Kinetic Isotope Effects.Acc Chem Res. 2018 Sep 18;51(9):1966-1974. doi: 10.1021/acs.accounts.8b00226. Epub 2018 Aug 28. Acc Chem Res. 2018. PMID: 30152685 Free PMC article. Review.
-
Environmentally coupled hydrogen tunneling. Linking catalysis to dynamics.Eur J Biochem. 2002 Jul;269(13):3113-21. doi: 10.1046/j.1432-1033.2002.03022.x. Eur J Biochem. 2002. PMID: 12084051 Review.
Cited by
-
Mg2+ binds to the surface of thymidylate synthase and affects hydride transfer at the interior active site.J Am Chem Soc. 2013 May 22;135(20):7583-92. doi: 10.1021/ja400761x. Epub 2013 May 10. J Am Chem Soc. 2013. PMID: 23611499 Free PMC article.
-
Hydrogen donor-acceptor fluctuations from kinetic isotope effects: a phenomenological model.Biochemistry. 2012 Aug 28;51(34):6860-70. doi: 10.1021/bi300613e. Epub 2012 Aug 15. Biochemistry. 2012. PMID: 22857146 Free PMC article.
-
Hydrogen tunneling in adenosylcobalamin-dependent glutamate mutase: evidence from intrinsic kinetic isotope effects measured by intramolecular competition.Biochemistry. 2010 Apr 13;49(14):3168-73. doi: 10.1021/bi1001695. Biochemistry. 2010. PMID: 20225826 Free PMC article.
-
A twin-track approach has optimized proton and hydride transfer by dynamically coupled tunneling during the evolution of protochlorophyllide oxidoreductase.J Biol Chem. 2011 Apr 1;286(13):11849-54. doi: 10.1074/jbc.M111.219626. Epub 2011 Feb 11. J Biol Chem. 2011. PMID: 21317291 Free PMC article.
-
Role of dynamics in enzyme catalysis: substantial versus semantic controversies.Acc Chem Res. 2015 Feb 17;48(2):466-73. doi: 10.1021/ar500322s. Epub 2014 Dec 24. Acc Chem Res. 2015. PMID: 25539442 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

