Protein tyrosine phosphatase SHP-2: a proto-oncogene product that promotes Ras activation
- PMID: 19622105
- PMCID: PMC11158110
- DOI: 10.1111/j.1349-7006.2009.01257.x
Protein tyrosine phosphatase SHP-2: a proto-oncogene product that promotes Ras activation
Abstract
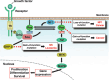
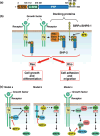
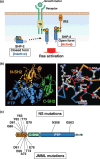
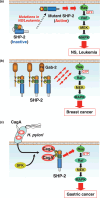
SHP-2 is a cytoplasmic protein tyrosine phosphatase (PTP) that contains two Src homology 2 (SH2) domains. Although PTPs are generally considered to be negative regulators on the basis of their ability to oppose the effects of protein tyrosine kinases, SHP-2 is unusual in that it promotes the activation of the Ras-MAPK signaling pathway by receptors for various growth factors and cytokines. The molecular basis for the activation of SHP-2 is also unique: In the basal state, the NH(2)-terminal SH2 domain of SHP-2 interacts with the PTP domain, resulting in autoinhibition of PTP activity; the binding of SHP-2 via its SH2 domains to tyrosine-phosphorylated growth factor receptors or docking proteins, however, results in disruption of this intramolecular interaction, leading to exposure of the PTP domain and catalytic activation. Indeed, SHP-2 proteins with artificial mutations in the NH(2)-terminal SH2 domain have been shown to act as dominant active mutants in vitro. Such activating mutations of PTPN11 (human SHP-2 gene) were subsequently identified in individuals with Noonan syndrome, a human developmental disorder that is sometimes associated with juvenile myelomonocytic leukemia. Furthermore, somatic mutations of PTPN11 were found to be associated with pediatric leukemia. SHP-2 is also thought to participate in the development of other malignant disorders, but in a manner independent of such activating mutations. Biochemical and functional studies of SHP-2 and genetic analysis of PTPN11 in human disorders have thus converged to provide new insight into the pathogenesis of cancer as well as potential new targets for cancer treatment.
Figures
Similar articles
-
Noonan syndrome-associated SHP2/PTPN11 mutants cause EGF-dependent prolonged GAB1 binding and sustained ERK2/MAPK1 activation.Hum Mutat. 2004 Mar;23(3):267-77. doi: 10.1002/humu.20005. Hum Mutat. 2004. PMID: 14974085
-
Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis.Blood. 2004 Mar 15;103(6):2325-31. doi: 10.1182/blood-2003-09-3287. Epub 2003 Nov 26. Blood. 2004. PMID: 14644997
-
The novel role of the C-terminal region of SHP-2. Involvement of Gab1 and SHP-2 phosphatase activity in Elk-1 activation.J Biol Chem. 2002 Aug 9;277(32):29330-41. doi: 10.1074/jbc.M112450200. Epub 2002 May 14. J Biol Chem. 2002. PMID: 12011040
-
New and Unexpected Biological Functions for the Src-Homology 2 Domain-Containing Phosphatase SHP-2 in the Gastrointestinal Tract.Cell Mol Gastroenterol Hepatol. 2015 Nov 14;2(1):11-21. doi: 10.1016/j.jcmgh.2015.11.001. eCollection 2016 Jan. Cell Mol Gastroenterol Hepatol. 2015. PMID: 28174704 Free PMC article. Review.
-
The tyrosine phosphatase Shp2 (PTPN11) in cancer.Cancer Metastasis Rev. 2008 Jun;27(2):179-92. doi: 10.1007/s10555-008-9126-y. Cancer Metastasis Rev. 2008. PMID: 18286234 Review.
Cited by
-
Recent advances in the discovery of protein tyrosine phosphatase SHP2 inhibitors.RSC Med Chem. 2022 Jan 15;13(3):246-257. doi: 10.1039/d1md00386k. eCollection 2022 Mar 23. RSC Med Chem. 2022. PMID: 35434626 Free PMC article. Review.
-
Role of the Tyrosine Phosphatase SHP-2 in Mediating Adrenomedullin Proangiogenic Activity in Solid Tumors.Front Oncol. 2021 Oct 8;11:753244. doi: 10.3389/fonc.2021.753244. eCollection 2021. Front Oncol. 2021. PMID: 34692535 Free PMC article.
-
Toward a More Precise Future for Oncology.Cancer Cell. 2020 Apr 13;37(4):431-442. doi: 10.1016/j.ccell.2020.03.014. Cancer Cell. 2020. PMID: 32289268 Free PMC article.
-
Inhibition of SHP2-mediated dephosphorylation of Ras suppresses oncogenesis.Nat Commun. 2015 Nov 30;6:8859. doi: 10.1038/ncomms9859. Nat Commun. 2015. PMID: 26617336 Free PMC article.
-
PD-1 and cancer: molecular mechanisms and polymorphisms.Immunogenetics. 2018 Feb;70(2):73-86. doi: 10.1007/s00251-017-1015-5. Epub 2017 Jun 22. Immunogenetics. 2018. PMID: 28642997 Review.
References
-
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer 2007; 7: 295–308. - PubMed
-
- Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer 2003; 3: 459–65. - PubMed
-
- Egan SE, Weinberg RA. The pathway to signal achievement. Nature 1993; 365: 781–3. - PubMed
-
- Takai Y, Sasaki T, Matozaki T. Small GTP‐binding proteins. Physiol Rev 2001; 81: 153–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

