Diet synergistically affects helicobacter pylori-induced gastric carcinogenesis in nonhuman primates
- PMID: 19622359
- PMCID: PMC2774828
- DOI: 10.1053/j.gastro.2009.07.041
Diet synergistically affects helicobacter pylori-induced gastric carcinogenesis in nonhuman primates
Abstract
Background & aims: Gastric cancer results from a combination of Helicobacter pylori (H pylori) infection, exposure to dietary carcinogens, and predisposing genetic make-up. Because the role of these factors in gastric carcinogenesis cannot be determined readily in human beings, the present study examined the role of an oral carcinogen and H pylori infection in rhesus monkeys.
Methods: Gastroscopies were performed in 23 monkeys assigned to 4 groups: controls; nitrosating carcinogen ethyl-nitro-nitrosoguanidine administration alone; inoculation of a virulent H pylori strain alone (H); and ethyl-nitro-nitrosoguanidine in combination with H pylori (EH). Follow-up gastroscopies and biopsies were performed at 3-month intervals for 5 years for pathologic and molecular studies.
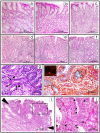
Results: Postinoculation, H and EH groups showed persistent infection and antral gastritis. Starting at 2 and 5 years, respectively, gastric intestinal metaplasia and intraepithelial neoplasia developed in 3 EH monkeys but in no other groups. Transcriptional analysis of biopsy specimens at 5 years revealed group-specific expression profiles, with striking changes in EH monkeys, plus a neoplasia-specific expression profile characterized by changes in multiple cancer-associated genes. Importantly, this neoplastic profile was evident in nonneoplastic mucosa, suggesting that the identified genes may represent markers preceding cancer.
Conclusions: Gastric intraglandular neoplasia is induced in primates when H pylori infection is associated with consumption of a carcinogen similar to the nitrosamines found in pickled vegetables, suggesting that H pylori and the carcinogen synergistically induce gastric neoplasia in primates.
Figures



References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Haenszel W, Correa P. Developments in the epidemiology of stomach cancer over the past decade. Cancer Res. 1975;35:3452–3459. - PubMed
-
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. - PubMed
-
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

