Effectiveness of quinine versus artemether-lumefantrine for treating uncomplicated falciparum malaria in Ugandan children: randomised trial
- PMID: 19622553
- PMCID: PMC2714631
- DOI: 10.1136/bmj.b2763
Effectiveness of quinine versus artemether-lumefantrine for treating uncomplicated falciparum malaria in Ugandan children: randomised trial
Abstract
Objective: To compare the effectiveness of oral quinine with that of artemether-lumefantrine in treating uncomplicated malaria in children.
Design: Randomised, open label effectiveness study.
Setting: Outpatient clinic of Uganda's national referral hospital in Kampala.
Participants: 175 children aged 6 to 59 months with uncomplicated malaria.
Interventions: Participants were randomised to receive oral quinine or artemether-lumefantrine administered by care givers at home.
Main outcome measures: Primary outcomes were parasitological cure rates after 28 days of follow-up unadjusted and adjusted by genotyping to distinguish recrudescence from new infections. Secondary outcomes were adherence to study drug, presence of gametocytes, recovery of haemoglobin concentration from baseline at day 28, and safety profiles.
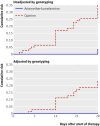
Results: Using survival analysis the cure rate unadjusted by genotyping was 96% for the artemether-lumefantrine group compared with 64% for the quinine group (hazard ratio 10.7, 95% confidence interval 3.3 to 35.5, P=0.001). In the quinine group 69% (18/26) of parasitological failures were due to recrudescence compared with none in the artemether-lumefantrine group. The mean adherence to artemether-lumefantrine was 94.5% compared with 85.4% to quinine (P=0.0008). Having adherence levels of 80% or more was associated with a decreased risk of treatment failure (0.44, 0.19 to 1.02, P=0.06). Adverse events did not differ between the two groups.
Conclusions: The effectiveness of a seven day course of quinine for the treatment of uncomplicated malaria in Ugandan children was significantly lower than that of artemether-lumefantrine. These findings question the advisability of the recommendation for quinine therapy for uncomplicated malaria in Africa.
Trial registration: ClinicalTrials.gov NCT00540202.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
Oral quinine for the treatment of uncomplicated malaria.BMJ. 2009 Jul 21;339:b2066. doi: 10.1136/bmj.b2066. BMJ. 2009. PMID: 19622550 No abstract available.
-
Quinine for malaria in children. Increasing adherence to quinine.BMJ. 2009 Aug 18;339:b3349. doi: 10.1136/bmj.b3349. BMJ. 2009. PMID: 19690009 No abstract available.
References
-
- World Health Organization. Antimalarial drug combination therapy. Report of WHO technical consultation. WHO/CDS/RBM/2001.35. Geneva: WHO, 2001.
-
- Meshnick SR, Dobson MJ. The history of antimalarial drugs. Antimalarial chemotherapy: mechanisms of action, resistance, and new directions in drug discovery. In: Rosenthal PJ, ed. Totowa, NJ: Humana Press, 2001:15-25.
-
- World Health Organization. WHO guidelines for the treatment of malaria. WHO/HTM/MAL/2006.Geneva: WHO, 2006:1108.
-
- World Health Organization.Global antimalarial drug policies database—AFRO. Antimalarial treatment policies for P falciparum and P vivax by country in WHO Africa region. www.who.int/malaria/amdp-afro.html (accessed 9 Sep 2008).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical