The titin-telethonin complex is a directed, superstable molecular bond in the muscle Z-disk
- PMID: 19622741
- PMCID: PMC2726412
- DOI: 10.1073/pnas.0902312106
The titin-telethonin complex is a directed, superstable molecular bond in the muscle Z-disk
Abstract
Mechanical stability of bonds and protein interactions has recently become accessible through single molecule mechanical experiments. So far, mechanical information about molecular bond mechanics has been largely limited to a single direction of force application. However, mechanical force acts as a vector in space and hence mechanical stability should depend on the direction of force application. In skeletal muscle, the giant protein titin is anchored in the Z-disk by telethonin. Much of the structural integrity of the Z-disk hinges upon the titin-telethonin bond. In this paper we show that the complex between the muscle proteins titin and telethonin forms a highly directed molecular bond. It is designed to resist ultra-high forces if they are applied in the direction along which it is loaded under physiological conditions, while it breaks easily along other directions. Highly directed molecular bonds match in an ideal way the requirements of tissues subject to mechanical stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
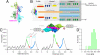


Comment in
-
A new direction for titin pulling.Proc Natl Acad Sci U S A. 2009 Aug 11;106(32):13149-50. doi: 10.1073/pnas.0906989106. Epub 2009 Aug 5. Proc Natl Acad Sci U S A. 2009. PMID: 19666561 Free PMC article. No abstract available.
References
-
- Tskhovrebova L, Trinick J. Titin: Properties and family relationships. Nat Rev Mol Cell Biol. 2003;4:679–689. - PubMed
-
- Labeit S, Kolmerer B. Titins: Giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270:293–296. - PubMed
-
- Maruyama K. Connectin/titin, giant elastic protein of muscle. FASEB J. 1997;11:341–345. - PubMed
-
- Li H, et al. Reverse engineering of the giant muscle protein titin. Nature. 2002;418:998–1002. - PubMed
-
- Mayans O, et al. Structural basis for activation of the titin kinase domain during myofibrillogenesis. Nature. 1998;395:863–869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

