Immunity to murine prostatic tumors: continuous provision of T-cell help prevents CD8 T-cell tolerance and activates tumor-infiltrating dendritic cells
- PMID: 19622771
- PMCID: PMC2732120
- DOI: 10.1158/0008-5472.CAN-08-4516
Immunity to murine prostatic tumors: continuous provision of T-cell help prevents CD8 T-cell tolerance and activates tumor-infiltrating dendritic cells
Retraction in
-
Retraction: Immunity to Murine Prostatic Tumors: Continuous Provision of T-Cell Help Prevents CD8 T-Cell Tolerance and Activates Tumor-Infiltrating Dendritic Cells.Cancer Res. 2016 Apr 15;76(8):2490. doi: 10.1158/0008-5472.CAN-16-0505. Epub 2016 Mar 24. Cancer Res. 2016. PMID: 27013202 Free PMC article. No abstract available.
Abstract
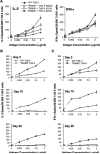
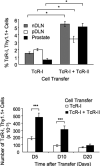
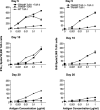
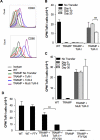
We reported previously that tumor-specific CD8(+) T cells (TcR-I) become tolerant in the transgenic adenocarcinoma of the mouse prostate (TRAMP) model. In this study, we show that CD4(+) TcR transgenic (TcR-II) T cells transferred into TRAMP mice became activated in lymph nodes, trafficked to the prostate, and initially functioned as T(H)1 cells. Although a single cotransfer of TcR-II cells delayed TcR-I cell tolerization, repeated transfer of TcR-II cells was required to prevent TcR-I cell tolerization and significantly slowed progression of TRAMP prostate tumors. After transfer of TcR-II cells, dendritic cells within the tumor expressed higher levels of costimulatory molecules and displayed an enhanced ability to stimulate proliferation of naive T cells. Blockade of CD40-CD40L interactions during TcR-II transfer resulted in a profound reduction in dendritic cell stimulatory capacity and a partial loss of TcR-I effector functions and tumor immunity. These data show that sustained provision of activated tumor-specific CD4(+) T cells alters the immunosuppressive tumor microenvironment, ultimately leading to the control of tumor growth. These findings will assist in the design of more effective immunotherapeutic approaches for cancer.
Figures

Similar articles
-
Prostate tumor microenvironment alters immune cells and prevents long-term survival in an orthotopic mouse model following flt3-ligand/CD40-ligand immunotherapy.J Immunother. 2004 Jan-Feb;27(1):13-26. doi: 10.1097/00002371-200401000-00002. J Immunother. 2004. PMID: 14676630
-
Anti-CD40 Antibody Fused to CD40 Ligand Is a Superagonist Platform for Adjuvant Intrinsic DC-Targeting Vaccines.Front Immunol. 2022 Jan 13;12:786144. doi: 10.3389/fimmu.2021.786144. eCollection 2021. Front Immunol. 2022. PMID: 35095862 Free PMC article.
-
CD4+ T cells elicit host immune responses to MHC class II-negative ovarian cancer through CCL5 secretion and CD40-mediated licensing of dendritic cells.J Immunol. 2010 May 15;184(10):5654-62. doi: 10.4049/jimmunol.0903247. Epub 2010 Apr 16. J Immunol. 2010. PMID: 20400704 Free PMC article.
-
CD40 and dendritic cell function.Crit Rev Immunol. 2003;23(1-2):83-107. doi: 10.1615/critrevimmunol.v23.i12.50. Crit Rev Immunol. 2003. PMID: 12906261 Review.
-
The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity.J Leukoc Biol. 2000 May;67(5):607-14. doi: 10.1002/jlb.67.5.607. J Leukoc Biol. 2000. PMID: 10810999 Review.
Cited by
-
Spontaneous presence of FOXO3-specific T cells in cancer patients.Oncoimmunology. 2014 Nov 14;3(8):e953411. doi: 10.4161/21624011.2014.953411. eCollection 2014. Oncoimmunology. 2014. PMID: 25960934 Free PMC article.
-
FOXO3: A master switch for regulating tolerance and immunity in dendritic cells.Oncoimmunology. 2012 Mar 1;1(2):252-254. doi: 10.4161/onci.1.2.18241. Oncoimmunology. 2012. Retraction in: Oncoimmunology. 2016 Apr 29;5(5):e1173506. doi: 10.1080/2162402X.2016.1173506. PMID: 22720261 Free PMC article. Retracted.
-
CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes.Cancer Res. 2010 Nov 1;70(21):8368-77. doi: 10.1158/0008-5472.CAN-10-1322. Epub 2010 Oct 12. Cancer Res. 2010. PMID: 20940398 Free PMC article.
-
Whole-body irradiation increases the magnitude and persistence of adoptively transferred T cells associated with tumor regression in a mouse model of prostate cancer.Cancer Immunol Res. 2014 Aug;2(8):777-88. doi: 10.1158/2326-6066.CIR-13-0164. Epub 2014 May 6. Cancer Immunol Res. 2014. PMID: 24801834 Free PMC article.
-
Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer.Science. 2014 May 9;344(6184):641-5. doi: 10.1126/science.1251102. Science. 2014. PMID: 24812403 Free PMC article.
References
-
- Chang AE, Yoshizawa H, Sakai K, Cameron MJ, Sondak VK, Shu S. Clinical observations on adoptive immunotherapy with vaccine-primed T-lymphocytes secondarily sensitized to tumor in vitro. Cancer Res. 1993;53:1043–50. - PubMed
-
- Ross S, Liu V, Abulafia R, Hogan C, Osband M. Adoptive immunotherapy of hormone-refractory, stage D2 prostate cancer using ex vivo activated autologous T cells (autolymphocyte therapy): results from a pilot study. Biotechnol Ther. 1993;4:197–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

