Second and third generation voltage-sensitive fluorescent proteins for monitoring membrane potential
- PMID: 19623246
- PMCID: PMC2706653
- DOI: 10.3389/neuro.02.005.2009
Second and third generation voltage-sensitive fluorescent proteins for monitoring membrane potential
Abstract
Over the last decade, optical neuroimaging methods have been enriched by engineered biosensors derived from fluorescent protein (FP) reporters fused to protein detectors that convert physiological signals into changes of intrinsic FP fluorescence. These FP-based indicators are genetically encoded, and hence targetable to specific cell populations within networks of heterologous cell types. Among this class of biosensors, the development of optical probes for membrane potential is both highly desirable and challenging. A suitable FP voltage sensor would indeed be a valuable tool for monitoring the activity of thousands of individual neurons simultaneously in a non-invasive manner. Previous prototypic genetically-encoded FP voltage indicators achieved a proof of principle but also highlighted several difficulties such as poor cell surface targeting and slow kinetics. Recently, we developed a new series of FRET-based Voltage-Sensitive Fluorescent Proteins (VSFPs), referred to as VSFP2s, with efficient targeting to the plasma membrane and high responsiveness to membrane potential signaling in excitable cells. In addition to these FRET-based voltage sensors, we also generated a third series of probes consisting of single FPs with response kinetics suitable for the optical imaging of fast neuronal signals. These newly available genetically-encoded reporters for membrane potential will be instrumental for future experimental approaches directed toward the understanding of neuronal network dynamics and information processing in the brain. Here, we review the development and current status of these novel fluorescent probes.
Keywords: fluorescence; fluorescent proteins; genetically-encoded voltage sensors; neuronal circuit dynamics; neurons; optical imaging; patch clamp.
Figures


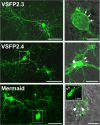
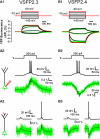


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

