Anchoring secreted proteins in endoplasmic reticulum by plant oleosin: the example of vitamin B12 cellular sequestration by transcobalamin
- PMID: 19623264
- PMCID: PMC2710007
- DOI: 10.1371/journal.pone.0006325
Anchoring secreted proteins in endoplasmic reticulum by plant oleosin: the example of vitamin B12 cellular sequestration by transcobalamin
Abstract
Background: Oleosin is a plant protein localized to lipid droplets and endoplasmic reticulum of plant cells. Our idea was to use it to target functional secretory proteins of interest to the cytosolic side of the endoplasmic reticulum of mammalian cells, through expressing oleosin-containing chimeras. We have designed this approach to create cellular models deficient in vitamin B12 (cobalamin) because of the known problematics associated to the obtainment of effective vitamin B12 deficient cell models. This was achieved by the overexpression of transcobalamin inside cells through anchoring to oleosin.
Methodology: chimera gene constructs including transcobalamin-oleosin (TC-O), green fluorescent protein-transcobalamin-oleosin (GFP-TC-O) and oleosin-transcobalamin (O-TC) were inserted into pAcSG2 and pCDNA3 vectors for expression in sf9 insect cells, Caco2 (colon carcinoma), NIE-115 (mouse neuroblastoma), HEK (human embryonic kidney), COS-7 (Green Monkey SV40-transfected kidney fibroblasts) and CHO (Chinese hamster ovary cells). The subcellular localization, the changes in vitamin B12 binding activity and the metabolic consequences were investigated in both Caco2 and NIE-115 cells.
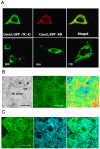
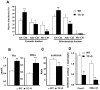
Principal findings: vitamin B12 binding was dramatically higher in TC-O than that in O-TC and wild type (WT). The expression of GFP-TC-O was observed in all cell lines and found to be co-localized with an ER-targeted red fluorescent protein and calreticulin of the endoplasmic reticulum in Caco2 and COS-7 cells. The overexpression of TC-O led to B12 deficiency, evidenced by impaired conversion of cyano-cobalamin to ado-cobalamin and methyl-cobalamin, decreased methionine synthase activity and reduced S-adenosyl methionine to S-adenosyl homocysteine ratio, as well as increases in homocysteine and methylmalonic acid concentration.
Conclusions/significance: the heterologous expression of TC-O in mammalian cells can be used as an effective strategy for investigating the cellular consequences of vitamin B12 deficiency. More generally, expression of oleosin-anchored proteins could be an interesting tool in cell engineering for studying proteins of pharmacological interest.
Conflict of interest statement
Figures




Similar articles
-
Vitamin B12-impaired metabolism produces apoptosis and Parkinson phenotype in rats expressing the transcobalamin-oleosin chimera in substantia nigra.PLoS One. 2009 Dec 21;4(12):e8268. doi: 10.1371/journal.pone.0008268. PLoS One. 2009. PMID: 20027219 Free PMC article.
-
Decreased vitamin B12 availability induces ER stress through impaired SIRT1-deacetylation of HSF1.Cell Death Dis. 2013 Mar 21;4(3):e553. doi: 10.1038/cddis.2013.69. Cell Death Dis. 2013. PMID: 23519122 Free PMC article.
-
Molecular and cellular effects of vitamin B12 in brain, myocardium and liver through its role as co-factor of methionine synthase.Biochimie. 2013 May;95(5):1033-40. doi: 10.1016/j.biochi.2013.01.020. Epub 2013 Feb 14. Biochimie. 2013. PMID: 23415654 Review.
-
Reduced vitamin B12 binding by transcobalamin II increases the risk of neural tube defects.QJM. 2001 Mar;94(3):159-66. doi: 10.1093/qjmed/94.3.159. QJM. 2001. PMID: 11259691
-
Neurological disorders in vitamin B12 deficiency.Ter Arkh. 2019 May 16;91(4):122-129. doi: 10.26442/00403660.2019.04.000116. Ter Arkh. 2019. PMID: 31094486 Review.
Cited by
-
Vitamin B₁₂-dependent taurine synthesis regulates growth and bone mass.J Clin Invest. 2014 Jul;124(7):2988-3002. doi: 10.1172/JCI72606. Epub 2014 Jun 9. J Clin Invest. 2014. PMID: 24911144 Free PMC article.
-
A lipid droplet protein of Nannochloropsis with functions partially analogous to plant oleosins.Plant Physiol. 2012 Apr;158(4):1562-9. doi: 10.1104/pp.111.193029. Epub 2012 Feb 3. Plant Physiol. 2012. PMID: 22307965 Free PMC article.
-
Vitamin B12 deficiency reduces proliferation and promotes differentiation of neuroblastoma cells and up-regulates PP2A, proNGF, and TACE.Proc Natl Acad Sci U S A. 2009 Dec 22;106(51):21930-5. doi: 10.1073/pnas.0811794106. Epub 2009 Dec 3. Proc Natl Acad Sci U S A. 2009. PMID: 19959661 Free PMC article.
-
Inherited disorders of cobalamin metabolism disrupt nucleocytoplasmic transport of mRNA through impaired methylation/phosphorylation of ELAVL1/HuR.Nucleic Acids Res. 2018 Sep 6;46(15):7844-7857. doi: 10.1093/nar/gky634. Nucleic Acids Res. 2018. PMID: 30016500 Free PMC article.
-
Vitamin B12-impaired metabolism produces apoptosis and Parkinson phenotype in rats expressing the transcobalamin-oleosin chimera in substantia nigra.PLoS One. 2009 Dec 21;4(12):e8268. doi: 10.1371/journal.pone.0008268. PLoS One. 2009. PMID: 20027219 Free PMC article.
References
-
- Cheng TL, Roffler S. Membrane-tethered proteins for basic research, imaging, and therapy. Med Res Rev. 2008;28:885–928. - PubMed
-
- Chou WC, Liao KW, Lo YC, Jiang SY, Yeh MY, et al. Expression of chimeric monomer and dimer proteins on the plasma membrane of mammalian cells. Biotechnol Bioeng. 1999;65:160–9. - PubMed
-
- Beaudoin F, Napier JA. The targeting and accumulation of ectopically expressed oleosin in non-seed tissues of Arabidopsis thaliana. Planta. 2000;210:439–45. - PubMed
-
- Capuano F, Beaudoin F, Napier JA, Shewry PR. Properties and exploitation of oleosins. Biotechnol Adv. 2007;25:203–6. - PubMed
-
- Wahlroos T, Soukka J, Denesyuk A, Wahlroos R, Korpela T, et al. Oleosin expression and trafficking during oil body biogenesis in tobacco leaf cells. Genesis. 2003;35:125–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

